Bifidobacterium and enteral feeding in preterm infants: cluster-randomized trial
- PMID: 24617812
- PMCID: PMC4285294
- DOI: 10.1111/ped.12330
Bifidobacterium and enteral feeding in preterm infants: cluster-randomized trial
Abstract
Background: This study evaluated the benefit of Bifidobacterium bifidum OLB6378 (B. bifidum) in very low-birthweight (VLBW) infants (birthweight <1500 g) for the acceleration of enteral feeding.
Methods: A cluster-randomized, double-blind, placebo-controlled trial was conducted in 19 hospitals, divided into two groups: the B group (n = 10 hospitals; B. bifidum given to infants within 48 h of birth) and the P group (n = 9 hospitals; infants received a placebo). The primary outcome was establishment of enteral feeding after birth, defined as the postnatal day at which enteral feeding exceeded 100 mL/(kg/day). Secondary outcomes were defined as incidence of morbidity and somatic growth before discharge.
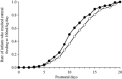
Results: Overall, 283 VLBW infants were enrolled in the study: B group, n = 153; and P group, n = 130. Enteral feeding was established within 21 days after birth in 233 infants, of whom 119 received B. bifidum and 114 received placebo until their bodyweight reached 2000 g. Enteral feeding was established significantly earlier in the B group, at 11.0 ± 3.6 days versus 12.1 ± 3.8 days in P group (P < 0.05). Infant growth during the stay in the neonatal intensive care unit was not different between groups, but the incidence of late-onset sepsis among all enrolled infants was significantly lower in the B group (3.9%, 6/153) than in the P group (10.0%, 13/130; P < 0.05). No differences were observed in the incidence of other adverse outcomes including mortality.
Conclusions: B. bifidum in VLBW infants accelerated the establishment of enteral feeding after birth without increasing the incidence of adverse effects.
Keywords: establishment of enteral feeding; necrotizing enterocolitis; neonate; probiotics; sepsis.
© 2014 The Authors. Pediatrics International published by Wiley Publishing Asia Pty Ltd on behalf of Japan Pediatric Society.
Figures
Similar articles
-
Effect of Bifidobacterium administration on very-low-birthweight infants.Pediatr Int. 2012 Oct;54(5):651-6. doi: 10.1111/j.1442-200X.2012.03649.x. Epub 2012 Jul 10. Pediatr Int. 2012. PMID: 22507386 Clinical Trial.
-
Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial.Pediatrics. 2008 Oct;122(4):693-700. doi: 10.1542/peds.2007-3007. Pediatrics. 2008. PMID: 18829790 Clinical Trial.
-
Oral supplementation with probiotics in very-low-birth-weight preterm infants: a randomized, double-blind, placebo-controlled trial.Am J Clin Nutr. 2009 Jun;89(6):1828-35. doi: 10.3945/ajcn.2008.26919. Epub 2009 Apr 15. Am J Clin Nutr. 2009. PMID: 19369375 Clinical Trial.
-
Probiotics for prevention of necrotizing enterocolitis in preterm infants.Evid Based Child Health. 2014 Sep;9(3):584-671. doi: 10.1002/ebch.1976. Evid Based Child Health. 2014. PMID: 25236307 Review.
-
Early enteral feeding in preterm infants.Semin Perinatol. 2019 Nov;43(7):151159. doi: 10.1053/j.semperi.2019.06.007. Epub 2019 Jul 24. Semin Perinatol. 2019. PMID: 31443906 Review.
Cited by
-
[Efficacy of probiotics in preventing late-onset sepsis in very low birth weight infants: a Meta analysis].Zhongguo Dang Dai Er Ke Za Zhi. 2021 Jun;23(6):599-607. doi: 10.7499/j.issn.1008-8830.2012014. Zhongguo Dang Dai Er Ke Za Zhi. 2021. PMID: 34130782 Free PMC article. Chinese.
-
Effect of probiotics on necrotizing enterocolitis in preterm infants: a network meta-analysis of randomized controlled trials.BMC Pediatr. 2025 Mar 27;25(1):237. doi: 10.1186/s12887-025-05469-z. BMC Pediatr. 2025. PMID: 40140789 Free PMC article.
-
Probiotics and the development of very low birthweight infants: follow-up study of a randomised trial.BMJ Paediatr Open. 2018 Apr 17;2(1):e000256. doi: 10.1136/bmjpo-2018-000256. eCollection 2018. BMJ Paediatr Open. 2018. PMID: 29687082 Free PMC article.
-
AGA Technical Review on the Role of Probiotics in the Management of Gastrointestinal Disorders.Gastroenterology. 2020 Aug;159(2):708-738.e4. doi: 10.1053/j.gastro.2020.05.060. Epub 2020 Jun 9. Gastroenterology. 2020. PMID: 32531292 Free PMC article. No abstract available.
-
Probiotic supplementation in preterm infants does not affect the risk of retinopathy of prematurity: a meta-analysis of randomized controlled trials.Sci Rep. 2017 Oct 12;7(1):13014. doi: 10.1038/s41598-017-13465-2. Sci Rep. 2017. PMID: 29026199 Free PMC article.
References
-
- Cilieborg MS, Boye M, Sangild PT. Bacterial colonization and gut development in preterm neonates. Early Hum. Dev. 2012;88:S41–49. - PubMed
-
- Westerbeek EA, van den Berg A, Lafeber HN, et al. The intestinal bacterial colonisation in preterm infants: A review of the literature. Clin. Nutr. 2006;25:361–368. - PubMed
-
- Alfaleh K, Anabrees J, Bassler D, et al. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst. Rev. 2011;(3):CD005496. - PubMed
-
- Yamasaki C, Totsu S, Uchiyama A, et al. Effect of Bifidobacterium administration on very-low-birthweight infants. Pediatr. Int. 2012;54:651–656. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources