Blood brain barrier and neuroinflammation are critical targets of IGF-1-mediated neuroprotection in stroke for middle-aged female rats
- PMID: 24618563
- PMCID: PMC3949985
- DOI: 10.1371/journal.pone.0091427
Blood brain barrier and neuroinflammation are critical targets of IGF-1-mediated neuroprotection in stroke for middle-aged female rats
Abstract
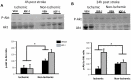
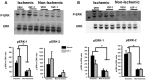
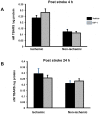
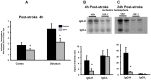
Ischemia-induced cerebral infarction is more severe in older animals as compared to younger animals, and is associated with reduced availability of insulin-like growth factor (IGF)-1. This study determined the effect of post-stroke IGF-1 treatment, and used microRNA profiling to identify mechanisms underlying IGF-1's neuroprotective actions. Post-stroke ICV administration of IGF-1 to middle-aged female rats reduced infarct volume by 39% when measured 24h later. MicroRNA analyses of ischemic tissue collected at the early post-stroke phase (4h) indicated that 8 out of 168 disease-related miRNA were significantly downregulated by IGF-1. KEGG pathway analysis implicated these miRNA in PI3K-Akt signaling, cell adhesion/ECM receptor pathways and T-and B-cell signaling. Specific components of these pathways were subsequently analyzed in vehicle and IGF-1 treated middle-aged females. Phospho-Akt was reduced by ischemia at 4h, but elevated by IGF-1 treatment at 24h. IGF-1 induced Akt activation was preceded by a reduction of blood brain barrier permeability at 4h post-stroke and global suppression of cytokines including IL-6, IL-10 and TNF-α. A subset of these cytokines including IL-6 was also suppressed by IGF-1 at 24h post-stroke. These data are the first to show that the temporal and mechanistic components of post-stroke IGF-1 treatment in older animals, and that cellular components of the blood brain barrier may serve as critical targets of IGF-1 in the aging brain.
Conflict of interest statement
Figures



References
-
- Towfighi A, Saver JL, Engelhardt R, Ovbiagele B (2007) A midlife stroke surge among women in the United States. Neurology 69: 1898–1904. - PubMed
-
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, et al. (2008) Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117: e25–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

