A generic method for design of oligomer-specific antibodies
- PMID: 24618582
- PMCID: PMC3949727
- DOI: 10.1371/journal.pone.0090857
A generic method for design of oligomer-specific antibodies
Abstract
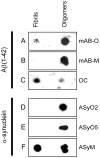
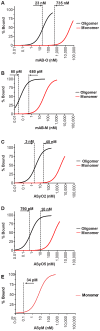
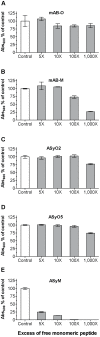
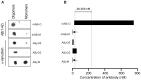
Antibodies that preferentially and specifically target pathological oligomeric protein and peptide assemblies, as opposed to their monomeric and amyloid counterparts, provide therapeutic and diagnostic opportunities for protein misfolding diseases. Unfortunately, the molecular properties associated with oligomer-specific antibodies are not well understood, and this limits targeted design and development. We present here a generic method that enables the design and optimisation of oligomer-specific antibodies. The method takes a two-step approach where discrimination between oligomers and fibrils is first accomplished through identification of cryptic epitopes exclusively buried within the structure of the fibrillar form. The second step discriminates between monomers and oligomers based on differences in avidity. We show here that a simple divalent mode of interaction, as within e.g. the IgG isotype, can increase the binding strength of the antibody up to 1500 times compared to its monovalent counterpart. We expose how the ability to bind oligomers is affected by the monovalent affinity and the turnover rate of the binding and, importantly, also how oligomer specificity is only valid within a specific concentration range. We provide an example of the method by creating and characterising a spectrum of different monoclonal antibodies against both the Aβ peptide and α-synuclein that are associated with Alzheimer's and Parkinson's diseases, respectively. The approach is however generic, does not require identification of oligomer-specific architectures, and is, in essence, applicable to all polypeptides that form oligomeric and fibrillar assemblies.
Conflict of interest statement
Figures
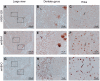

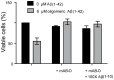
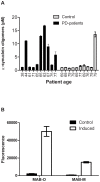
References
-
- Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, et al. (2012) Amyloid fibril protein nomenclature: 2012 recommendations from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid 19: 167–170. - PubMed
-
- Lasagna-Reeves CA, Castillo-Carranza DL, Guerrero-Muoz MJ, Jackson GR, Kayed R (2010) Preparation and characterization of neurotoxic tau oligomers. Biochemistry 49: 10039–10041. - PubMed
-
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, et al. (2002) Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416: 535–539. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

