Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy
- PMID: 24618797
- PMCID: PMC4151501
- DOI: 10.1227/NEU.0000000000000343
Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy
Abstract
Background: Open surgery effectively treats mesial temporal lobe epilepsy, but carries the risk of neurocognitive deficits, which may be reduced with minimally invasive alternatives.
Objective: To describe technical and clinical outcomes of stereotactic laser amygdalohippocampotomy with real-time magnetic resonance thermal imaging guidance.
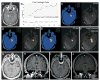
Methods: With patients under general anesthesia and using standard stereotactic methods, 13 adult patients with intractable mesial temporal lobe epilepsy (with and without mesial temporal sclerosis [MTS]) prospectively underwent insertion of a saline-cooled fiberoptic laser applicator in amygdalohippocampal structures from an occipital trajectory. Computer-controlled laser ablation was performed during continuous magnetic resonance thermal imaging followed by confirmatory contrast-enhanced anatomic imaging and volumetric reconstruction. Clinical outcomes were determined from seizure diaries.
Results: A mean 60% volume of the amygdalohippocampal complex was ablated in 13 patients (9 with MTS) undergoing 15 procedures. Median hospitalization was 1 day. With follow-up ranging from 5 to 26 months (median, 14 months), 77% (10/13) of patients achieved meaningful seizure reduction, of whom 54% (7/13) were free of disabling seizures. Of patients with preoperative MTS, 67% (6/9) achieved seizure freedom. All recurrences were observed before 6 months. Variances in ablation volume and length did not account for individual clinical outcomes. Although no complications of laser therapy itself were observed, 1 significant complication, a visual field defect, resulted from deviated insertion of a stereotactic aligning rod, which was corrected before ablation.
Conclusion: Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy is a technically novel, safe, and effective alternative to open surgery. Further evaluation with larger cohorts over time is warranted.
Conflict of interest statement
The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
Figures




Comment in
-
Journal Club: Real-Time Magnetic Resonance-Guided Stereotactic Laser Amygdalohippocampotomy for Mesial Temporal Lobe Epilepsy.Neurosurgery. 2015 Aug;77(2):307-9. doi: 10.1227/NEU.0000000000000817. Neurosurgery. 2015. PMID: 26181781 No abstract available.
-
Response to Journal Club: Real-time Magnetic Resonance-Guided Stereotactic Laser Amygdalohippocampotomy for Mesial Temporal Lobe Epilepsy.Neurosurgery. 2015 Sep;77(3):E502-4. doi: 10.1227/NEU.0000000000000876. Neurosurgery. 2015. PMID: 26280828 No abstract available.
References
-
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. - PubMed
-
- Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7:525–537. - PubMed
-
- Lutz MT, Clusmann H, Elger CE, Schramm J, Helmstaedter C. Neuropsychological outcome after selective amygdalohippocampectomy with transsylvian versus transcortical approach: a randomized prospective clinical trial of surgery for temporal lobe epilepsy. Epilepsia. 2004;45:809–816. - PubMed
-
- Von Rhein B, et al. Neuropsychological outcome after selective amygdalohippocampectomy: subtemporal versus transsylvian approach. J Neurol Neurosurg Psychiatr. 2012;83:887–893. - PubMed
-
- Crane J, Milner B. Do I know you? Face perception and memory in patients with selective amygdalo-hippocampectomy. Neuropsychologia. 2002;40:530–538. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

