Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response
- PMID: 24618995
- PMCID: PMC3950222
- DOI: 10.1371/journal.pone.0091574
Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response
Abstract
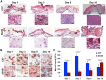
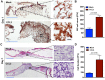
Prior studies suggest that the impaired healing seen in diabetic wounds derives from a state of persistent hyper-inflammation characterized by harmful increases in inflammatory leukocytes including macrophages. However, such studies have focused on wounds at later time points (day 10 or older), and very little attention has been given to the dynamics of macrophage responses in diabetic wounds early after injury. Given the importance of macrophages for the process of healing, we studied the dynamics of macrophage response during early and late phases of healing in diabetic wounds. Here, we report that early after injury, the diabetic wound exhibits a significant delay in macrophage infiltration. The delay in the macrophage response in diabetic wounds results from reduced Chemokine (C-C motif) ligand 2 (CCL2) expression. Importantly, one-time treatment with chemoattractant CCL2 significantly stimulated healing in diabetic wounds by restoring the macrophage response. Our data demonstrate that, rather than a hyper-inflammatory state; the early diabetic wound exhibits a paradoxical and damaging decrease in essential macrophage response. Our studies suggest that the restoration of the proper kinetics of macrophage response may be able to jumpstart subsequent healing stages. CCL2 chemokine-based therapy may be an attractive strategy to promote healing in diabetic wounds.
Conflict of interest statement
Figures


References
-
- Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341: 738–746. - PubMed
-
- Bjarnsholt T, Kirketerp-Moller K, Jensen PO, Madsen KG, Phipps R, et al. (2008) Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen 16: 2–10. - PubMed
-
- Donath MY, Shoelson SE (2011) Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11: 98–107. - PubMed
-
- Adzick NS, Harrison MR, Glick PL, Beckstead JH, Villa RL, et al. (1985) Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations. J Pediatr Surg 20: 315–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

