Water diffusion reveals networks that modulate multiregional morphological plasticity after repetitive brain stimulation
- PMID: 24619090
- PMCID: PMC3970501
- DOI: 10.1073/pnas.1320223111
Water diffusion reveals networks that modulate multiregional morphological plasticity after repetitive brain stimulation
Abstract
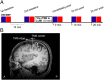
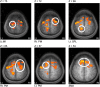
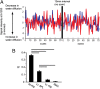
Repetitive brain stimulation protocols induce plasticity in the stimulated site in brain slice models. Recent evidence from network models has indicated that additional plasticity-related changes occur in nonstimulated remote regions. Despite increasing use of brain stimulation protocols in experimental and clinical settings, the neural substrates underlying the additional effects in remote regions are unknown. Diffusion-weighted MRI (DWI) probes water diffusion and can be used to estimate morphological changes in cortical tissue that occur with the induction of plasticity. Using DWI techniques, we estimated morphological changes induced by application of repetitive transcranial magnetic stimulation (rTMS) over the left primary motor cortex (M1). We found that rTMS altered water diffusion in multiple regions including the left M1. Notably, the change in water diffusion was retained longest in the left M1 and remote regions that had a correlation of baseline fluctuations in water diffusion before rTMS. We conclude that synchronization of water diffusion at rest between stimulated and remote regions ensures retention of rTMS-induced changes in water diffusion in remote regions. Synchronized fluctuations in the morphology of cortical microstructures between stimulated and remote regions might identify networks that allow retention of plasticity-related morphological changes in multiple regions after brain stimulation protocols. These results increase our understanding of the effects of brain stimulation-induced plasticity on multiregional brain networks. DWI techniques could provide a tool to evaluate treatment effects of brain stimulation protocols in patients with brain disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hallett M, Chokroverty S (2005) Magnetic Stimulation in Clinical Neurophysiology (Elsevier Butterworth–Heinemann, Philadelphia), 2nd Ed.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

