Separation of on-target efficacy from adverse effects through rational design of a bitopic adenosine receptor agonist
- PMID: 24619092
- PMCID: PMC3970544
- DOI: 10.1073/pnas.1320962111
Separation of on-target efficacy from adverse effects through rational design of a bitopic adenosine receptor agonist
Abstract
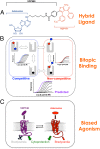
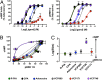
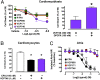
The concepts of allosteric modulation and biased agonism are revolutionizing modern approaches to drug discovery, particularly in the field of G protein-coupled receptors (GPCRs). Both phenomena exploit topographically distinct binding sites to promote unique GPCR conformations that can lead to different patterns of cellular responsiveness. The adenosine A1 GPCR (A1AR) is a major therapeutic target for cardioprotection, but current agents acting on the receptor are clinically limited for this indication because of on-target bradycardia as a serious adverse effect. In the current study, we have rationally designed a novel A1AR ligand (VCP746)--a hybrid molecule comprising adenosine linked to a positive allosteric modulator--specifically to engender biased signaling at the A1AR. We validate that the interaction of VCP746 with the A1AR is consistent with a bitopic mode of receptor engagement (i.e., concomitant association with orthosteric and allosteric sites) and that the compound displays biased agonism relative to prototypical A1AR ligands. Importantly, we also show that the unique pharmacology of VCP746 is (patho)physiologically relevant, because the compound protects against ischemic insult in native A1AR-expressing cardiomyoblasts and cardiomyocytes but does not affect rat atrial heart rate. Thus, this study provides proof of concept that bitopic ligands can be designed as biased agonists to promote on-target efficacy without on-target side effects.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5(12):993–996. - PubMed
-
- Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7(4):339–357. - PubMed
-
- Jacoby E, Bouhelal R, Gerspacher M, Seuwen K. The 7 TM G-protein-coupled receptor target family. ChemMedChem. 2006;1(8):761–782. - PubMed
-
- Christopoulos A. Allosteric binding sites on cell-surface receptors: Novel targets for drug discovery. Nat Rev Drug Discov. 2002;1(3):198–210. - PubMed
-
- May LT, Leach K, Sexton PM, Christopoulos A. Allosteric modulation of G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2007;47(1):1–51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

