Identification of the major ACE-inhibitory peptides produced by enzymatic hydrolysis of a protein concentrate from cuttlefish wastewater
- PMID: 24619242
- PMCID: PMC3967217
- DOI: 10.3390/md12031390
Identification of the major ACE-inhibitory peptides produced by enzymatic hydrolysis of a protein concentrate from cuttlefish wastewater
Abstract
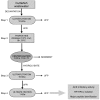
The aim of this work was the purification and identification of the major angiotensin converting enzyme (ACE) inhibitory peptides produced by enzymatic hydrolysis of a protein concentrate recovered from a cuttlefish industrial manufacturing effluent. This process consisted on the ultrafiltration of cuttlefish softening wastewater, with a 10 kDa cut-off membrane, followed by the hydrolysis with alcalase of the retained fraction. Alcalase produced ACE inhibitors reaching the highest activity (IC₅₀ = 76.8 ± 15.2 μg mL⁻¹) after 8 h of proteolysis. Sequential ultrafiltration of the 8 h hydrolysate with molecular weight cut-off (MWCO) membranes of 10 and 1 kDa resulted in the increased activity of each permeate, with a final IC₅₀ value of 58.4 ± 4.6 μg mL⁻¹. Permeate containing peptides lower than 1 kDa was separated by reversed-phase high performance liquid chromatography (RP-HPLC). Four fractions (A-D) with potent ACE inhibitory activity were isolated and their main peptides identified using high performance liquid chromatography coupled to an electrospray ion trap Fourier transform ion cyclotron resonance-mass spectrometer (HPLC-ESI-IT-FTICR) followed by comparison with databases and de novo sequencing. The amino acid sequences of the identified peptides contained at least one hydrophobic and/or a proline together with positively charged residues in at least one of the three C-terminal positions. The IC₅₀ values of the fractions ranged from 1.92 to 8.83 μg mL⁻¹, however this study fails to identify which of these peptides are ultimately responsible for the potent antihypertensive activity of these fractions.
Figures



References
-
- World Health Organization . Causes of Death 2008: Data Sources and Methods. World Health Organization; Geneva, Switzerland: 2011. [(accessed on 20 October 2013)]. Available online: http://www.who.int/healthinfo/global_burden_disease/cod_2008_sources_met....
-
- World Health Organization . A Global Brief on Hypertension. Silent Killer, Global Public Health Crisis. World Health Organization; Geneva, Switzerland: 2013. [(accessed on 14 December 2013)]. Available online: http://apps.who.int/iris/bitstream/10665/79059/1/WHO_DCO_WHD_2013.2_eng.pdf.
-
- Lim S.S., Vos T., Flaxman A.D., Danaei G., Shibuya K., Adair-Rohani H., Al Mazroa M.A., Amann M., Anderson H.R., Andrews K.G., et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

