Assessment of circulating microRNAs in plasma of lung cancer patients
- PMID: 24619302
- PMCID: PMC6272001
- DOI: 10.3390/molecules19033038
Assessment of circulating microRNAs in plasma of lung cancer patients
Abstract
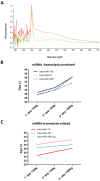
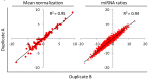
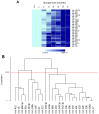
Lung cancer is the most common cause of cancer deaths worldwide and numerous ongoing research efforts are directed to identify new strategies for its early detection. The development of non-invasive blood-based biomarkers for cancer detection in its preclinical phases is crucial to improve the outcome of this deadly disease. MicroRNAs (miRNAs) are a new promising class of circulating biomarkers for cancer detection and prognosis definition, but lack of consensus on data normalization methods for circulating miRNAs and the critical issue of haemolysis, has affected the identification of circulating miRNAs with diagnostic potential. We describe here an interesting approach for profiling circulating miRNAs in plasma samples based on the evaluation of reciprocal miRNA levels measured by quantitative Real-Time PCR. By monitoring changes of plasma miRNA-ratios, it is possible to assess the deregulation of tumor-related miRNAs and identify signatures with diagnostic and prognostic value. In addition, to avoid bias due to the release of miRNAs from blood cells, a miRNA-ratios signature distinguishing haemolyzed samples was identified. The method described was validated in plasma samples of lung cancer patients, but given its reproducibility and reliability, could be potentially applied for the identification of diagnostic circulating miRNAs in other diseases.
Conflict of interest statement
Gabriella Sozzi, Mattia Boeri, and Ugo Pastorino are coinventors on two patent applications regarding the miRNA signatures disclosed in this article.
Figures
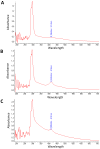
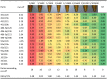
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

