Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial
- PMID: 24621228
- PMCID: PMC4233950
- DOI: 10.1111/epi.12534
Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial
Abstract
Objective: To demonstrate the safety and effectiveness of responsive stimulation at the seizure focus as an adjunctive therapy to reduce the frequency of seizures in adults with medically intractable partial onset seizures arising from one or two seizure foci.
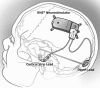
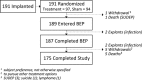
Methods: Randomized multicenter double-blinded controlled trial of responsive focal cortical stimulation (RNS System). Subjects with medically intractable partial onset seizures from one or two foci were implanted, and 1 month postimplant were randomized 1:1 to active or sham stimulation. After the fifth postimplant month, all subjects received responsive stimulation in an open label period (OLP) to complete 2 years of postimplant follow-up.
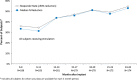
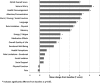
Results: All 191 subjects were randomized. The percent change in seizures at the end of the blinded period was -37.9% in the active and -17.3% in the sham stimulation group (p = 0.012, Generalized Estimating Equations). The median percent reduction in seizures in the OLP was 44% at 1 year and 53% at 2 years, which represents a progressive and significant improvement with time (p < 0.0001). The serious adverse event rate was not different between subjects receiving active and sham stimulation. Adverse events were consistent with the known risks of an implanted medical device, seizures, and of other epilepsy treatments. There were no adverse effects on neuropsychological function or mood.
Significance: Responsive stimulation to the seizure focus reduced the frequency of partial-onset seizures acutely, showed improving seizure reduction over time, was well tolerated, and was acceptably safe. The RNS System provides an additional treatment option for patients with medically intractable partial-onset seizures.
Keywords: Cortical stimulation; Focal seizures; Neurostimulator; Partial seizures; Responsive stimulation.
© 2014 The Authors. Epilepsia published by Wiley Periodicals, Inc. on behalf of International League Against Epilepsy.
Figures


Comment in
-
The NeuroPace trial: missing knowledge and insights.Epilepsia. 2014 Sep;55(9):1469-70. doi: 10.1111/epi.12701. Epilepsia. 2014. PMID: 25223508 No abstract available.
References
-
- Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. - PubMed
-
- IOM (Institute of Medicine) Epilepsy Across the Spectrum: Promoting Health and Understanding. Washington, DC: The National Academies Press; 2012. - PubMed
-
- Morrell MJ. RNS® system in Epilepsy Study Group Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. - PubMed
-
- Devinsky O, Vickrey BG, Cramer J, et al. Development of the quality of life in epilepsy inventory. Epilepsia. 1995;36:1089–1104. - PubMed
-
- Borghs S, de la Loge C, Cramer JA. Defining minimally important change in QOLIE-31 scores: estimates from three placebo-controlled lacosamide trials in patients with partial-onset seizures. Epilepsy Behav. 2012;23:230–234. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

