Ligand-controlled asymmetric arylation of aliphatic α-amino anion equivalents
- PMID: 24621247
- PMCID: PMC3985922
- DOI: 10.1021/ja501560x
Ligand-controlled asymmetric arylation of aliphatic α-amino anion equivalents
Abstract
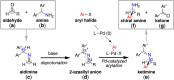
A palladium-catalyzed asymmetric arylation of 9-aminofluorene-derived imines using a chiral dialkylbiaryl phosphine as the supporting ligand has been developed. This transformation allows for enantioselective access to a diverse range of α-branched benzylamines.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

