Dynamics between actin and the VE-cadherin/catenin complex: novel aspects of the ARP2/3 complex in regulation of endothelial junctions
- PMID: 24621569
- PMCID: PMC4049858
- DOI: 10.4161/cam.28243
Dynamics between actin and the VE-cadherin/catenin complex: novel aspects of the ARP2/3 complex in regulation of endothelial junctions
Abstract
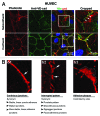
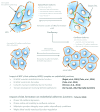
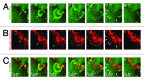
Endothelial adherens junctions are critical for physiological and pathological processes such as differentiation, maintenance of entire monolayer integrity, and the remodeling. The endothelial-specific VE-cadherin/catenin complex provides the backbone of adherens junctions and acts in close interaction with actin filaments and actin/myosin-mediated contractility to fulfill the junction demands. The functional connection between the cadherin/catenin complex and actin filaments might be either directly through ?-catenins, or indirectly e.g., via linker proteins such as vinculin, p120ctn, ?-actinin, or EPLIN. However, both junction integrity and dynamic remodeling have to be contemporarily coordinated. The actin-related protein complex ARP2/3 and its activating molecules, such as N-WASP and WAVE, have been shown to regulate the lammellipodia-mediated formation of cell junctions in both epithelium and endothelium. Recent reports now demonstrate a novel aspect of the ARP2/3 complex and the nucleating-promoting factors in the maintenance of endothelial barrier function and junction remodeling of established endothelial cell junctions. Those mechanisms open novel possibilities; not only in fulfilling physiological demands but obtained information may be of critical importance in pathologies such as wound healing, angiogenesis, inflammation, and cell diapedesis.
Keywords: ARP2/3 complex; VE-cadherin; actin; cortical actin; endothelium; stress fibers.
Figures

References
-
- Dejana E, Vestweber D. The Role VE-Cadherin in Vascular Morphogenesis and Permeability Control. In: VanRoy F, ed. Molecular Biology of Cadherins, 2013:119-44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
