New miRNA profiles accurately distinguish renal cell carcinomas and upper tract urothelial carcinomas from the normal kidney
- PMID: 24621579
- PMCID: PMC3951427
- DOI: 10.1371/journal.pone.0091646
New miRNA profiles accurately distinguish renal cell carcinomas and upper tract urothelial carcinomas from the normal kidney
Erratum in
- PLoS One. 2014;9(6):e100063
Abstract
Background: Upper tract urothelial carcinomas (UT-UC) can invade the pelvicalyceal system making differential diagnosis of the various histologically distinct renal cell carcinoma (RCC) subtypes and UT-UC, difficult. Correct diagnosis is critical for determining appropriate surgery and post-surgical treatments. We aimed to identify microRNA (miRNA) signatures that can accurately distinguish the most prevalent RCC subtypes and UT-UC form the normal kidney.
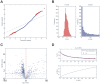
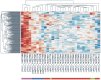
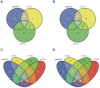
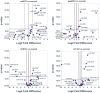
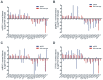
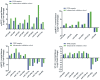
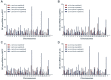
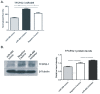
Methods and findings: miRNA profiling was performed on FFPE tissue sections from RCC and UT-UC and normal kidney and 434 miRNAs were significantly deregulated in cancerous vs. the normal tissue. Hierarchical clustering distinguished UT-UCs from RCCs and classified the various RCC subtypes among them. qRT-PCR validated the deregulated expression profile for the majority of the miRNAs and ROC analysis revealed their capability to discriminate between tumour and normal kidney. An independent cohort of freshly frozen RCC and UT-UC samples was used to validate the deregulated miRNAs with the best discriminatory ability (AUC>0.8, p<0.001). Many of them were located within cytogenetic regions that were previously reported to be significantly aberrated. miRNA targets were predicted using the miRWalk algorithm and ingenuity pathway analysis identified the canonical pathways and curated networks of the deregulated miRNAs. Using the miRWalk algorithm, we further identified the top anti-correlated mRNA/miRNA pairs, between the deregulated miRNAs from our study and the top co-deregulated mRNAs among 5 independent ccRCC GEO datasets. The AB8/13 undifferentiated podocyte cells were used for functional assays using luciferase reporter constructs and the developmental transcription factor TFCP2L1 was proved to be a true target of miR-489, which was the second most upregulated miRNA in ccRCC.
Conclusions: We identified novel miRNAs specific for each RCC subtype and UT-UC, we investigated their putative targets, the networks and pathways in which they participate and we functionally verified the true targets of the top deregulated miRNAs.
Conflict of interest statement
Figures





References
-
- Meloni-Ehrig AM (2002) Renal cancer: cytogenetic and molecular genetic aspects. Am J Med Genet 115: 164–172. - PubMed
-
- Ficarra V, Martignoni G, Galfano A, Novara G, Gobbo S, et al. (2006) Prognostic role of the histologic subtypes of renal cell carcinoma after slide revision. Eur Urol 50: 786–793. - PubMed
-
- Lopez-Beltran A, Kirkali Z, Cheng L, Egevad L, Regueiro JC, et al. (2008) Targeted therapies and biological modifiers in urologic tumors: pathobiology and clinical implications. Semin Diagn Pathol 25: 232–244. - PubMed
-
- Tazi el M, Essadi I, Tazi MF, Ahellal Y, M'Rabti H, et al. (2011) Advanced treatments in non-clear renal cell carcinoma. Urol J 8: 1–11. - PubMed
-
- Youssef YM, White NMA, Grigull J, Krizova A, Samy C, et al. (2011) Accurate molecular classification of kidney cancer subtypes using microRNA signature. Eur Urol 59: 721–730. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

