Combination therapy of human umbilical cord blood cells and granulocyte colony stimulating factor reduces histopathological and motor impairments in an experimental model of chronic traumatic brain injury
- PMID: 24621603
- PMCID: PMC3951247
- DOI: 10.1371/journal.pone.0090953
Combination therapy of human umbilical cord blood cells and granulocyte colony stimulating factor reduces histopathological and motor impairments in an experimental model of chronic traumatic brain injury
Abstract
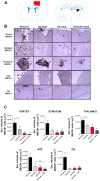
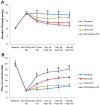
Traumatic brain injury (TBI) is associated with neuro-inflammation, debilitating sensory-motor deficits, and learning and memory impairments. Cell-based therapies are currently being investigated in treating neurotrauma due to their ability to secrete neurotrophic factors and anti-inflammatory cytokines that can regulate the hostile milieu associated with chronic neuroinflammation found in TBI. In tandem, the stimulation and mobilization of endogenous stem/progenitor cells from the bone marrow through granulocyte colony stimulating factor (G-CSF) poses as an attractive therapeutic intervention for chronic TBI. Here, we tested the potential of a combined therapy of human umbilical cord blood cells (hUCB) and G-CSF at the acute stage of TBI to counteract the progressive secondary effects of chronic TBI using the controlled cortical impact model. Four different groups of adult Sprague Dawley rats were treated with saline alone, G-CSF+saline, hUCB+saline or hUCB+G-CSF, 7-days post CCI moderate TBI. Eight weeks after TBI, brains were harvested to analyze hippocampal cell loss, neuroinflammatory response, and neurogenesis by using immunohistochemical techniques. Results revealed that the rats exposed to TBI treated with saline exhibited widespread neuroinflammation, impaired endogenous neurogenesis in DG and SVZ, and severe hippocampal cell loss. hUCB monotherapy suppressed neuroinflammation, nearly normalized the neurogenesis, and reduced hippocampal cell loss compared to saline alone. G-CSF monotherapy produced partial and short-lived benefits characterized by low levels of neuroinflammation in striatum, DG, SVZ, and corpus callosum and fornix, a modest neurogenesis, and a moderate reduction of hippocampal cells loss. On the other hand, combined therapy of hUCB+G-CSF displayed synergistic effects that robustly dampened neuroinflammation, while enhancing endogenous neurogenesis and reducing hippocampal cell loss. Vigorous and long-lasting recovery of motor function accompanied the combined therapy, which was either moderately or short-lived in the monotherapy conditions. These results suggest that combined treatment rather than monotherapy appears optimal for abrogating histophalogical and motor impairments in chronic TBI.
Conflict of interest statement
Figures


References
-
- Faul M, Xu L, Wald MM, Coronado VG (2010) Traumatic brain injury in the United States: Emergency department visits, hospitalizations and deaths. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control
-
- Fabrizio KS, Keltner NL (2010) Traumatic brain injury in operation enduring freedom/operation iraqi freedom: a primer. The Nursing Clinics of North America 45: 569–580, vi. - PubMed
-
- Ettenhofer KS, Abeles N (2009) The significant of mild traumatic brain injury to cognition and self-reported sympons in long term recovery from injury. J Clin Exp Neuropsychol 31 (3) 363–72. - PubMed
-
- Ozen LJ, Fernandes MA (2012) Slowing down after a mild traumatic brain injury: a strategy to improve cognitive task performance? Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists 27: 85–100. - PubMed
-
- Werner C, Engelhard K (2007) Pathophysiology of traumatic brain injury. British Journal of Anaesthesia 99: 4–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

