Protein carbonylation and heat shock proteins in human skeletal muscle: relationships to age and sarcopenia
- PMID: 24621945
- PMCID: PMC4301601
- DOI: 10.1093/gerona/glu007
Protein carbonylation and heat shock proteins in human skeletal muscle: relationships to age and sarcopenia
Abstract
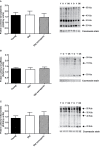
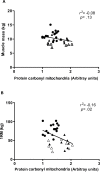
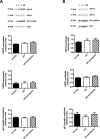
Aging is associated with a gradual loss of muscle mass termed sarcopenia, which has significant impact on quality-of-life. Because oxidative stress is proposed to negatively impact upon musculoskeletal aging, we investigated links between human aging and markers of oxidative stress, and relationships to muscle mass and strength in young and old nonsarcopenic and sarcopenic adults. Sixteen young and 16 old males (further subdivided into "old" and "old sarcopenic") were studied. The abundance of protein carbonyl adducts within skeletal muscle sarcoplasmic, myofibrillar, and mitochondrial protein subfractions from musculus vastus lateralis biopsies were determined using Oxyblot immunoblotting techniques. In addition, concentrations of recognized cytoprotective proteins (eg, heat shock proteins [HSP], αβ-crystallin) were also assayed. Aging was associated with increased mitochondrial (but not myofibrillar or sarcoplasmic) protein carbonyl adducts, independently of (stage-I) sarcopenia. Correlation analyses of all subjects revealed that mitochondrial protein carbonyl abundance negatively correlated with muscle strength ([1-repetition maximum], p = .02, r (2) = -.16), but not muscle mass (p = .13, r (2) = -.08). Abundance of cytoprotective proteins, including various HSPs (HSP 27 and 70), were unaffected by aging/sarcopenia. To conclude, these data reveal that mitochondrial protein carbonylation increases moderately with age, and that this increase may impact upon skeletal muscle function, but is not a hallmark of (stage-I) sarcopenia, per se.
Keywords: Carbonylation; Heat shock protein.; Mitochondria; Sarcopenia.
© The Author 2014. Published by Oxford University Press on behalf of The Gerontological Society of America.
Figures


References
-
- Rosenberg I. Summary comments: epidemiological and methodological problems in determining nutritional status of older persons. Am J Clin Nutr. 1989;50:1231–3.
-
- Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010;95:139–159. 10.1093/bmb/ldq008. - PubMed
-
- Rennie MJ, Selby A, Atherton P, et al. Facts, noise and wishful thinking: muscle protein turnover in aging and human disuse atrophy. Scand J Med Sci Sports. 2010;20:5–9. 10.1111/j.1600-0838.2009.00967.x. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

