A mixed incoherent feed-forward loop allows conditional regulation of response dynamics
- PMID: 24621982
- PMCID: PMC3951346
- DOI: 10.1371/journal.pone.0091243
A mixed incoherent feed-forward loop allows conditional regulation of response dynamics
Abstract
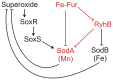
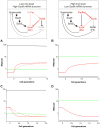
Expression of the SodA superoxide dismutase (MnSOD) in Escherichia coli is regulated by superoxide concentration through the SoxRS system and also by Fur (Ferric uptake regulator) through a mixed incoherent feed forward loop (FFL) containing the RyhB small regulatory RNA. In this work I theoretically analyze the function of this feed forward loop as part of the network controlling expression of the two cytoplasmic superoxide dismutases, SodA and SodB. I find that feed forward regulation allows faster response to superoxide stress at low intracellular iron levels compared to iron rich conditions. That is, it can conditionally modulate the response time of a superimposed transcriptional control mechanism.
Conflict of interest statement
Figures


Similar articles
-
Control of Escherichia coli superoxide dismutase (sodA and sodB) genes by the ferric uptake regulation (fur) locus.J Bacteriol. 1990 Apr;172(4):1930-8. doi: 10.1128/jb.172.4.1930-1938.1990. J Bacteriol. 1990. PMID: 2180912 Free PMC article.
-
Fur positive regulation of iron superoxide dismutase in Escherichia coli: functional analysis of the sodB promoter.J Bacteriol. 2000 Jul;182(13):3802-8. doi: 10.1128/JB.182.13.3802-3808.2000. J Bacteriol. 2000. PMID: 10850997 Free PMC article.
-
Regulatory roles of Fnr, Fur, and Arc in expression of manganese-containing superoxide dismutase in Escherichia coli.Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3217-21. doi: 10.1073/pnas.89.8.3217. Proc Natl Acad Sci U S A. 1992. PMID: 1565612 Free PMC article.
-
Regulation of sod genes in Escherichia coli: relevance to superoxide dismutase function.Mol Microbiol. 1991 Nov;5(11):2599-610. doi: 10.1111/j.1365-2958.1991.tb01968.x. Mol Microbiol. 1991. PMID: 1779751 Review.
-
Roles of manganese and iron in the regulation of the biosynthesis of manganese-superoxide dismutase in Escherichia coli.FEMS Microbiol Rev. 1994 Aug;14(4):315-23. doi: 10.1111/j.1574-6976.1994.tb00105.x. FEMS Microbiol Rev. 1994. PMID: 7917419 Review.
Cited by
-
Increasing dissolved-oxygen disrupts iron homeostasis in production cultures of Escherichia coli.Antonie Van Leeuwenhoek. 2017 Jan;110(1):115-124. doi: 10.1007/s10482-016-0781-7. Epub 2016 Oct 18. Antonie Van Leeuwenhoek. 2017. PMID: 27757702 Free PMC article.
-
Involvement of catalase and superoxide dismutase in hydrophobic organic solvent tolerance of Escherichia coli.AMB Express. 2021 Jun 29;11(1):97. doi: 10.1186/s13568-021-01258-w. AMB Express. 2021. PMID: 34189628 Free PMC article.
-
Function and mechanism of action of the small regulatory RNA ArcZ in Enterobacterales.RNA. 2024 Aug 16;30(9):1107-1121. doi: 10.1261/rna.080010.124. RNA. 2024. PMID: 38839110 Free PMC article. Review.
-
The Leucine-Responsive Regulatory Protein Lrp Participates in Virulence Regulation Downstream of Small RNA ArcZ in Erwinia amylovora.mBio. 2019 May 28;10(3):e00757-19. doi: 10.1128/mBio.00757-19. mBio. 2019. PMID: 31138749 Free PMC article.
-
Battle for Metals: Regulatory RNAs at the Front Line.Front Cell Infect Microbiol. 2022 Jul 5;12:952948. doi: 10.3389/fcimb.2022.952948. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35865816 Free PMC article. Review.
References
-
- Diaz JM, Hansel CM, Voelker BM, Mendes CM, Andeer PF, et al. (2013) Widespread production of extracellular superoxide by heterotrophic bacteria. Science 340: 1223–1226. - PubMed
-
- El Bekay R, Alvarez M, Carballo M, Martin-Nieto J, Monteseirin J, et al. (2002) Activation of phagocytic cell NADPH oxidase by norfloxacin: a potential mechanism to explain its bactericidal action. J Leukoc Biol 71: 255–261. - PubMed
-
- Benov LT, Fridovich I (1994) Escherichia coli expresses a copper- and zinc-containing superoxide dismutase. J Biol Chem 269: 25310–25314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

