Multilocus sequence typing of Mycoplasma hyorhinis strains identified by a real-time TaqMan PCR assay
- PMID: 24622092
- PMCID: PMC3993664
- DOI: 10.1128/JCM.03437-13
Multilocus sequence typing of Mycoplasma hyorhinis strains identified by a real-time TaqMan PCR assay
Abstract
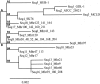
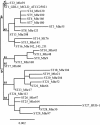
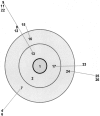
A real-time TaqMan PCR assay based on the gene encoding the protein p37 was developed to detect Mycoplasma hyorhinis. Its specificity was validated with 29 epidemiologically unrelated M. hyorhinis strains (28 field strains and one reference strain) and other mycoplasma species or with other microorganisms commonly found in pigs. The estimated detection limit of this qPCR assay was 125 microorganism equivalents/μl. The same 29 epidemiologically unrelated M. hyorhinis strains and four previously fully sequenced strains were typed by two portable typing methods, the sequencing of the p37 gene and a multilocus sequence typing (MLST) scheme. The first method revealed 18 distinct nucleotide sequences and insufficient discriminatory power (0.934). The MLST scheme was developed with the sequenced genomes of the M. hyorhinis strains HUB-1, GDL-1, MCLD, and SK76 and based on the genes dnaA, rpoB, gyrB, gltX, adk, and gmk. In total, 2,304 bp of sequence was analyzed for each strain. MLST was capable of subdividing the 33 strains into 29 distinct sequence types. The discriminatory power of the method was >0.95, which is the threshold value for interpreting typing results with confidence (D=0.989). Population analysis showed that recombination in M. hyorhinis occurs and that strains are diverse but with a certain clonality (one unique clonal complex was identified). The new qPCR assay and the robust MLST scheme are available for the acquisition of new knowledge on M. hyorhinis epidemiology. A web-accessible database has been set up for the M. hyorhinis MLST scheme at http://pubmlst.org/mhyorhinis/.
Figures
Similar articles
-
Multilocus sequence typing of Mycoplasma agalactiae.J Med Microbiol. 2011 Jun;60(Pt 6):803-811. doi: 10.1099/jmm.0.028159-0. Epub 2011 Mar 3. J Med Microbiol. 2011. PMID: 21372188
-
Genotyping Mycoplasma hyorhinis by multi-locus sequence typing and multiple-locus variable-number tandem-repeat analysis.Vet Microbiol. 2020 Oct;249:108836. doi: 10.1016/j.vetmic.2020.108836. Epub 2020 Sep 12. Vet Microbiol. 2020. PMID: 32956967
-
A core genome multilocus sequence typing scheme for Mycoplasma hyorhinis.Vet Microbiol. 2021 Nov;262:109249. doi: 10.1016/j.vetmic.2021.109249. Epub 2021 Oct 5. Vet Microbiol. 2021. PMID: 34628273
-
Development of a Multilocus Sequence Typing Scheme for Molecular Typing of Mycoplasma pneumoniae.J Clin Microbiol. 2015 Oct;53(10):3195-203. doi: 10.1128/JCM.01301-15. Epub 2015 Jul 22. J Clin Microbiol. 2015. PMID: 26202118 Free PMC article.
-
Genetic variability and limited clonality of Mycoplasma hyorhinis in pig herds.Vet Microbiol. 2016 Aug 15;191:9-14. doi: 10.1016/j.vetmic.2016.05.015. Epub 2016 May 26. Vet Microbiol. 2016. PMID: 27374901
Cited by
-
EAVLD 2024 - 7th Congress of the European Association of Veterinary Laboratory Diagnosticians.Ital J Food Saf. 2024 Dec 16;13(4):13488. doi: 10.4081/ijfs.2024.13488. eCollection 2024 Nov 12. Ital J Food Saf. 2024. PMID: 39829721 Free PMC article.
-
Molecular epidemiology of Mycoplasma hyorhinis porcine field isolates in the United States.PLoS One. 2019 Oct 21;14(10):e0223653. doi: 10.1371/journal.pone.0223653. eCollection 2019. PLoS One. 2019. PMID: 31634349 Free PMC article.
-
Bacterial polyarthritis in post-weaning pigs in a high-density swine breeding area in Italy.J Vet Diagn Invest. 2022 Jul;34(4):709-711. doi: 10.1177/10406387221090903. Epub 2022 May 20. J Vet Diagn Invest. 2022. PMID: 35593676 Free PMC article.
-
Association Between Infectious Agents and Lesions in Post-Weaned Piglets and Fattening Heavy Pigs With Porcine Respiratory Disease Complex (PRDC).Front Vet Sci. 2020 Sep 11;7:636. doi: 10.3389/fvets.2020.00636. eCollection 2020. Front Vet Sci. 2020. PMID: 33024748 Free PMC article.
-
Multilocus sequence typing characterizes diversity of Ureaplasma diversum strains, and intra-species variability induces different immune response profiles.BMC Vet Res. 2020 May 26;16(1):163. doi: 10.1186/s12917-020-02380-w. BMC Vet Res. 2020. PMID: 32456681 Free PMC article.
References
-
- Kobisch M, Friis N. 1996. Swine mycoplasmoses. Rev. Sci. Tech. 15:1569–1605 - PubMed
-
- Thacker EL. 2006. Mycoplasmal diseases, p 701–717 In Straw BE, Zimmerman J, D'Allaire S, Taylor DJ. (ed), Diseases of swine, 9th ed. Iowa State University Press, Ames, IA
-
- Sorensen V, Jorsal SE, Mousing J. 2006. Diseases of the respiratory system, p 149–177 In Straw BE, Zimmerman J, D'Allaire S, Taylor DJ. (ed), Diseases of swine, 9th ed. Iowa State University Press, Ames, IA
-
- Leneveu P, Robert N, Keïta A, Pagot E, Pommier P, Tessier P. 2005. Lung lesions in pigs at slaughter: a 2-year epidemiological study in France. Int. J. Appl. Res. Vet. Med. 3:259–265 http://www.jarvm.com/articles/Vol3Iss3/ISPAIA.pdf
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

