Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial
- PMID: 24622414
- PMCID: PMC3957186
- DOI: 10.1016/S2213-8587(13)70111-6
Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial
Abstract
Background: Type 1 diabetes results from autoimmune targeting of the pancreatic β cells, likely mediated by effector memory T (Tem) cells. CD2, a T cell surface protein highly expressed on Tem cells, is targeted by the fusion protein alefacept, depleting Tem cells and central memory T (Tcm) cells. We postulated that alefacept would arrest autoimmunity and preserve residual β cells in patients newly diagnosed with type 1 diabetes.
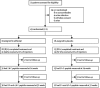
Methods: The T1DAL study is a phase 2, double-blind, placebo-controlled trial in patients with type 1 diabetes, aged 12-35 years who, within 100 days of diagnosis, were enrolled at 14 US sites. Patients were randomly assigned (2:1) to receive alefacept (two 12-week courses of 15 mg intramuscularly per week, separated by a 12-week pause) or a placebo. Randomisation was stratified by site, and was computer-generated with permuted blocks of three patients per block. All participants and site personnel were masked to treatment assignment. The primary endpoint was the change from baseline in mean 2 h C-peptide area under the curve (AUC) at 12 months. Secondary endpoints at 12 months were the change from baseline in the 4 h C-peptide AUC, insulin use, major hypoglycaemic events, and HbA1c concentrations. This trial is registered with ClinicalTrials.gov, number NCT00965458.
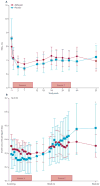
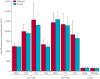
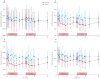
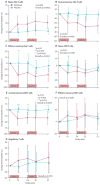
Findings: Of 73 patients assessed for eligibility, 33 were randomly assigned to receive alefacept and 16 to receive placebo. The mean 2 h C-peptide AUC at 12 months increased by 0.015 nmol/L (95% CI -0.080 to 0.110) in the alefacept group and decreased by 0.115 nmol/L (-0.278 to 0.047) in the placebo group, and the difference between groups was not significant (p=0.065). However, key secondary endpoints were met: the mean 4 h C-peptide AUC was significantly higher (mean increase of 0.015 nmol/L [95% CI -0.076 to 0.106] vs decrease of -0.156 nmol/L [-0.305 to -0.006]; p=0.019), and daily insulin use (0.48 units per kg per day for placebo vs 0.36 units per kg per day for alefacept; p=0.02) and the rate of hypoglycaemic events (mean of 10.9 events per person per year for alefacept vs 17.3 events for placebo; p<0.0001) was significantly lower at 12 months in the alefacept group than in the placebo group. Mean HbA1c concentrations at week 52 were not different between treatment groups (p=0.75). So far, no serious adverse events were reported and all patients had at least one adverse event. In the alefacept group, 29 (88%) participants had an adverse event related to study drug versus 15 (94%) participants in the placebo group. In the alefacept group, 14 (42%) participants had grade 3 or 4 adverse events compared with nine (56%) participants in the placebo group; no deaths occurred.
Interpretation: Although the primary outcome was not met, at 12 months, alefacept preserved the 4 h C-peptide AUC, lowered insulin use, and reduced hypoglycaemic events, suggesting efficacy. Safety and tolerability were similar in the alefacept and placebo groups. Alefacept could be useful to preserve β-cell function in patients with new-onset type 1 diabetes.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
No other potential conflicts of interest relevant to this article were reported.
Figures


Comment in
-
Restoring immune balance in type 1 diabetes.Lancet Diabetes Endocrinol. 2013 Dec;1(4):261-3. doi: 10.1016/S2213-8587(13)70123-2. Epub 2013 Sep 23. Lancet Diabetes Endocrinol. 2013. PMID: 24622404 No abstract available.
References
-
- Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26(3):832–6. Epub 2003/03/01. - PubMed
-
- Feutren G, Papoz L, Assan R, Vialettes B, Karsenty G, Vexiau P, et al. Cyclosporin increases the rate and length of remissions in insulin-dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet. 1986;2(8499):119–24. Epub 1986/07/19. - PubMed
-
- Cook JJ, Hudson I, Harrison LC, Dean B, Colman PG, Werther GA, et al. Double-blind controlled trial of azathioprine in children with newly diagnosed type I diabetes. Diabetes. 1989;38(6):779–83. - PubMed
-
- Chase HP, Butler-Simon N, Garg SK, Hayward A, Klingensmith GJ, Hamman RF, et al. Cyclosporine A for the treatment of new-onset insulin-dependent diabetes mellitus. Pediatrics. 1990;85(3):241–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- TR000006/TR/NCATS NIH HHS/United States
- N01 AI015416/AI/NIAID NIH HHS/United States
- UM2 AI117870/AI/NIAID NIH HHS/United States
- U01 AI101984/AI/NIAID NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- UL1TR000003/TR/NCATS NIH HHS/United States
- UL1RR024134/RR/NCRR NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- UL1 TR000442-06/TR/NCATS NIH HHS/United States
- UL1 TR000006/TR/NCATS NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- DP3 DK097681/DK/NIDDK NIH HHS/United States
- UL1 TR000442/TR/NCATS NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

