Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial
- PMID: 24622416
- PMCID: PMC6489466
- DOI: 10.1016/S2213-8587(13)70065-2
Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial
Abstract
Background: Type 1 diabetes results from T-cell-mediated destruction of β cells. Findings from preclinical studies and pilot clinical trials suggest that antithymocyte globulin (ATG) might be effective for reducing this autoimmune response. We assessed the safety and efficacy of rabbit ATG in preserving islet function in participants with recent-onset type 1 diabetes, and report here our 12-month results.
Methods: For this phase 2, randomised, placebo-controlled, clinical trial, we enrolled patients with recent-onset type 1 diabetes, aged 12-35 years, and with a peak C-peptide of 0.4 nM or greater on mixed meal tolerance test from 11 sites in the USA. We used a computer generated randomisation sequence to randomly assign patients (2:1, with permuted-blocks of size three or six and stratified by study site) to receive either 6.5 mg/kg ATG or placebo over a course of four days. All participants were masked and initially managed by an unmasked drug management team, which managed all aspects of the study until month 3. Thereafter, to maintain masking for diabetes management throughout the remainder of the study, participants received diabetes management from an independent, masked study physician and nurse educator. The primary endpoint was the baseline-adjusted change in 2-h area under the curve C-peptide response to mixed meal tolerance test from baseline to 12 months. Analyses were by intention to treat. This is a planned interim analysis of an on-going trial that will run for 24 months of follow-up. This study is registered with ClinicalTrials.gov, number NCT00515099.
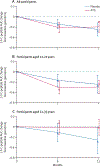
Findings: Between Sept 10, 2007, and June 1, 2011, we screened 154 individuals, randomly allocating 38 to ATG and 20 to placebo. We recorded no between-group difference in the primary endpoint: participants in the ATG group had a mean change in C-peptide area under the curve of -0.195 pmol/mL (95% CI -0.292 to -0.098) and those in the placebo group had a mean change of -0.239 pmol/mL (-0.361 to -0.118) in the placebo group (p=0.591). All except one participant in the ATG group had both cytokine release syndrome and serum sickness, which was associated with a transient rise in interleukin-6 and acute-phase proteins. Acute T cell depletion occurred in the ATG group, with slow reconstitution over 12 months. However, effector memory T cells were not depleted, and the ratio of regulatory to effector memory T cells declined in the first 6 months and stabilised thereafter. ATG-treated patients had 159 grade 3-4 adverse events, many associated with T-cell depletion, compared with 13 in the placebo group, but we detected no between-group difference in incidence of infectious diseases.
Interpretation: Our findings suggest that a brief course of ATG does not result in preservation of β-cell function 12 months later in patients with new-onset type 1 diabetes. Generalised T-cell depletion in the absence of specific depletion of effector memory T cells and preservation of regulatory T cells seems to be an ineffective treatment for type 1 diabetes.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of interest
SEG served as a consultant on an advisory board for Genzyme. All other authors declare that they have no conflicts of interest.
Figures






Comment in
-
ATG in type 1 diabetes: an unanswered question.Lancet Diabetes Endocrinol. 2013 Dec;1(4):265-6. doi: 10.1016/S2213-8587(13)70087-1. Epub 2013 Aug 28. Lancet Diabetes Endocrinol. 2013. PMID: 24622406 No abstract available.
References
-
- Imperatore G, Boyle JP, Thompson TJ, et al., and the SEARCH for Diabetes in Youth Study Group. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care 2012; 35: 2515–20. - PMC - PubMed
-
- Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, and the EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet 2009; 373: 2027–33. - PubMed
-
- Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003; 26: 832–36. - PubMed
-
- The Canadian-European Randomized Control Trial Group. Cyclosporin-induced remission of IDDM after early intervention. Association of 1 yr of cyclosporin treatment with enhanced insulin secretion. Diabetes 1988; 37: 1574–82. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

