TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling
- PMID: 24622514
- PMCID: PMC4181409
- DOI: 10.1126/scitranslmed.3006927
TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling
Abstract
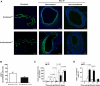
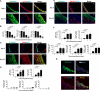
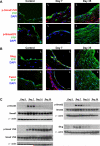
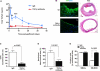
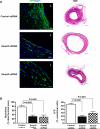
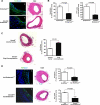
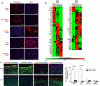
Veins grafted into an arterial environment undergo a complex vascular remodeling process. Pathologic vascular remodeling often results in stenosed or occluded conduit grafts. Understanding this complex process is important for improving the outcome of patients with coronary and peripheral artery disease undergoing surgical revascularization. Using in vivo murine cell lineage-tracing models, we show that endothelial-derived cells contribute to neointimal formation through endothelial-to-mesenchymal transition (EndMT), which is dependent on early activation of the Smad2/3-Slug signaling pathway. Antagonism of transforming growth factor-β (TGF-β) signaling by TGF-β neutralizing antibody, short hairpin RNA-mediated Smad3 or Smad2 knockdown, Smad3 haploinsufficiency, or endothelial cell-specific Smad2 deletion resulted in decreased EndMT and less neointimal formation compared to controls. Histological examination of postmortem human vein graft tissue corroborated the changes observed in our mouse vein graft model, suggesting that EndMT is operative during human vein graft remodeling. These data establish that EndMT is an important mechanism underlying neointimal formation in interpositional vein grafts, and identifies the TGF-β-Smad2/3-Slug signaling pathway as a potential therapeutic target to prevent clinical vein graft stenosis.
Figures
Comment in
-
Maladapted endothelial cells flip the mesenchymal switch.Sci Transl Med. 2014 Mar 12;6(227):227fs12. doi: 10.1126/scitranslmed.3008623. Sci Transl Med. 2014. PMID: 24622512
References
-
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011 Feb 1;123:e18. - PMC - PubMed
-
- Mehta RH, Ferguson TB, Lopes RD, Hafley GE, Mack MJ, Kouchoukos NT, Gibson CM, Harrington RA, Califf RM, Peterson ED, Alexander JH. Saphenous Vein Grafts With Multiple Versus Single Distal Targets in Patients Undergoing Coronary Artery Bypass Surgery: One-Year Graft Failure and Five-Year Outcomes From the Project of Ex-Vivo Vein Graft Engineering via Transfection (PREVENT) IV Trial. Circulation. 2011 Jul 19;124:280. - PMC - PubMed
-
- Mitra AK, Gangahar DM, Agrawal DK. Cellular, molecular and immunological mechanisms in the pathophysiology of vein graft intimal hyperplasia. Immunol Cell Biol. 2006 Apr;84:115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

