Silencing of hypoxia-inducible factor-1α gene attenuates chronic ischemic renal injury in two-kidney, one-clip rats
- PMID: 24623146
- PMCID: PMC4024731
- DOI: 10.1152/ajprenal.00673.2013
Silencing of hypoxia-inducible factor-1α gene attenuates chronic ischemic renal injury in two-kidney, one-clip rats
Abstract
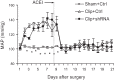
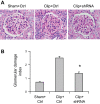
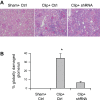
Overactivation of hypoxia-inducible factor (HIF)-1α is implicated as a pathogenic factor in chronic kidney diseases (CKD). However, controversy exists regarding the roles of HIF-1α in CKD. Additionally, although hypoxia and HIF-1α activation are observed in various CKD and HIF-1α has been shown to stimulate fibrogenic factors, there is no direct evidence whether HIF-1α is an injurious or protective factor in chronic renal hypoxic injury. The present study determined whether knocking down the HIF-1α gene can attenuate or exaggerate kidney damage using a chronic renal ischemic model. Chronic renal ischemia was induced by unilaterally clamping the left renal artery for 3 wk in Sprague-Dawley rats. HIF-1α short hairpin (sh) RNA or control vectors were transfected into the left kidneys. Experimental groups were sham+control vector, clip+control vector, and clip+HIF-1α shRNA. Enalapril was used to normalize blood pressure 1 wk after clamping the renal artery. HIF-1α protein levels were remarkably increased in clipped kidneys, and this increase was blocked by shRNA. Morphological examination showed that HIF-1α shRNA significantly attenuated injury in clipped kidneys: glomerular injury indices were 0.71 ± 0.04, 2.50 ± 0.12, and 1.34 ± 0.11, and the percentage of globally damaged glomeruli was 0.02, 34.3 ± 5.0, and 6.3 ± 1.6 in sham, clip, and clip+shRNA groups, respectively. The protein levels of collagen and α-smooth muscle actin also dramatically increased in clipped kidneys, but this effect was blocked by HIF-1α shRNA. In conclusion, long-term overactivation of HIF-1α is a pathogenic factor in chronic renal injury associated with ischemia/hypoxia.
Keywords: chronic kidney diseases; collagen; renal fibrosis; α-smooth muscle actin.
Copyright © 2014 the American Physiological Society.
Figures




References
-
- Atkinson AB, Brown JJ, Fraser R, Lever AF, Morton JJ, Riegger AJ, Robertson JI. Angiotensin II and renal hypertension in dog, rat and man: effect of converting enzyme inhibition. Clin Exp Hypertens 2: 499–524, 1980 - PubMed
-
- Burns KD, Li N. The role of angiotensin II-stimulated renal tubular transport in hypertension. Curr Hypertens Rep 5: 165–171, 2003 - PubMed
-
- Burns WC, Kantharidis P, Thomas MC. The role of tubular epithelial-mesenchymal transition in progressive kidney disease. Cells Tissues Organs 185: 222–231, 2007 - PubMed
-
- Chumakova OV, Liopo AV, Andreev VG, Cicenaite I, Evers BM, Chakrabarty S, Pappas TC, Esenaliev RO. Composition of PLGA and PEI/DNA nanoparticles improves ultrasound-mediated gene delivery in solid tumors in vivo. Cancer Lett 261: 215–225, 2008 - PubMed
-
- Dallatu MK, Choi M, Oyekan AO. Inhibition of prolyl hydroxylase domain-containing protein on hypertension/renal injury induced by high salt diet and nitric oxide withdrawal. J Hypertens, 2013 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

