A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks
- PMID: 24623156
- PMCID: PMC4341027
- DOI: 10.1007/s00429-014-0717-9
A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks
Abstract
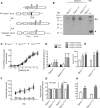
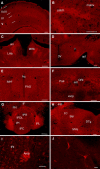
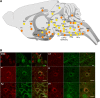
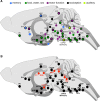
Opioid receptors are G protein-coupled receptors (GPCRs) that modulate brain function at all levels of neural integration, including autonomic, sensory, emotional and cognitive processing. Mu (MOR) and delta (DOR) opioid receptors functionally interact in vivo, but whether interactions occur at circuitry, cellular or molecular levels remains unsolved. To challenge the hypothesis of MOR/DOR heteromerization in the brain, we generated redMOR/greenDOR double knock-in mice and report dual receptor mapping throughout the nervous system. Data are organized as an interactive database offering an opioid receptor atlas with concomitant MOR/DOR visualization at subcellular resolution, accessible online. We also provide co-immunoprecipitation-based evidence for receptor heteromerization in these mice. In the forebrain, MOR and DOR are mainly detected in separate neurons, suggesting system-level interactions in high-order processing. In contrast, neuronal co-localization is detected in subcortical networks essential for survival involved in eating and sexual behaviors or perception and response to aversive stimuli. In addition, potential MOR/DOR intracellular interactions within the nociceptive pathway offer novel therapeutic perspectives.
Figures




References
-
- Befort K, Filliol D, Ghate A, Darcq E, Matifas A, Muller J et al (2008) Mu-opioid receptor activation induces transcriptional plasticity in the central extended amygdala. Eur J Neurosci 27(11):2973–2984 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

