Mitochondrial response to nutrient availability and its role in metabolic disease
- PMID: 24623376
- PMCID: PMC4023882
- DOI: 10.1002/emmm.201303782
Mitochondrial response to nutrient availability and its role in metabolic disease
Abstract
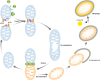
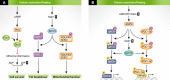
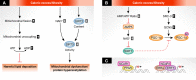
Metabolic inflexibility is defined as an impaired capacity to switch between different energy substrates and is a hallmark of insulin resistance and type 2 diabetes mellitus (T2DM). Hence, understanding the mechanisms underlying proper metabolic flexibility is key to prevent the development of metabolic disease and physiological deterioration. An important downstream player in the effects of metabolic flexibility is the mitochondrion. The objective of this review was to describe how mitochondrial metabolism adapts to limited nutrient situations or caloric excess by changes in mitochondrial function or biogenesis, as well as to define the mechanisms propelling these changes. Altogether, this should pinpoint key regulatory points by which metabolic flexibility might be ameliorated in situations of metabolic disease.
Figures
References
-
- Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, Daugaard JR, Lloberas J, Camps M, Zierath JR, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–17197. - PubMed
-
- Broskey NT, Greggio C, Boss A, Boutant M, Dwyer A, Schlueter L, Hans D, Gremion G, Kreis R, Boesch C, et al. Skeletal muscle mitochondria in the elderly: effects of physical fitness and exercise training. J Clin Endocrinol Metab. 2014 DOI: 10.1210/jc.2013-3983. - DOI - PubMed
-
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

