HIV-1 Vpu antagonism of tetherin inhibits antibody-dependent cellular cytotoxic responses by natural killer cells
- PMID: 24623433
- PMCID: PMC4093850
- DOI: 10.1128/JVI.00449-14
HIV-1 Vpu antagonism of tetherin inhibits antibody-dependent cellular cytotoxic responses by natural killer cells
Abstract
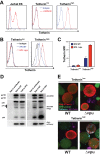
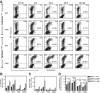
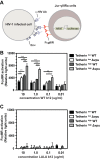
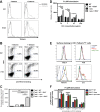
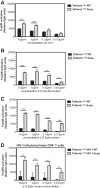
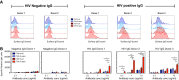
The type I interferon-inducible factor tetherin retains virus particles on the surfaces of cells infected with vpu-deficient human immunodeficiency virus type 1 (HIV-1). While this mechanism inhibits cell-free viral spread, the immunological implications of tethered virus have not been investigated. We found that surface tetherin expression increased the antibody opsonization of vpu-deficient HIV-infected cells. The absence of Vpu also stimulated NK cell-activating FcγRIIIa signaling and enhanced NK cell degranulation and NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC). The deletion of vpu in HIV-1-infected primary CD4(+) T cells enhanced the levels of antibody binding and Fc receptor signaling mediated by HIV-positive-patient-derived antibodies. The magnitudes of antibody binding and Fc signaling were both highly correlated to the levels of tetherin on the surfaces of infected primary CD4 T cells. The affinity of antibody binding to FcγRIIIa was also found to be critical in mediating efficient Fc activation. These studies implicate Vpu antagonism of tetherin as an ADCC evasion mechanism that prevents antibody-mediated clearance of virally infected cells.
Importance: The ability of the HIV-1 accessory factor to antagonize tetherin has been considered to primarily function by limiting the spread of virus by preventing the release of cell-free virus. This study supports the hypothesis that a major function of Vpu is to decrease the recognition of infected cells by anti-HIV antibodies at the cell surface, thereby reducing recognition by antibody-dependent clearance by natural killer cells.
Figures


References
-
- Sedaghat AR, German J, Teslovich TM, Cofrancesco J, Jr, Jie CC, Talbot CC, Jr, Siliciano RF. 2008. Chronic CD4+ T-cell activation and depletion in human immunodeficiency virus type 1 infection: type I interferon-mediated disruption of T-cell dynamics. J. Virol. 82:1870–1883. 10.1128/JVI.02228-07 - DOI - PMC - PubMed
-
- Hyrcza MD, Kovacs C, Loutfy M, Halpenny R, Heisler L, Yang S, Wilkins O, Ostrowski M, Der SD. 2007. Distinct transcriptional profiles in ex vivo CD4+ and CD8+ T cells are established early in human immunodeficiency virus type 1 infection and are characterized by a chronic interferon response as well as extensive transcriptional changes in CD8+ T cells. J. Virol. 81:3477–3486. 10.1128/JVI.01552-06 - DOI - PMC - PubMed
-
- Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245–252. 10.1016/j.chom.2008.03.001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

