Reinitiation after translation of two upstream open reading frames (ORF) governs expression of the ORF35-37 Kaposi's sarcoma-associated herpesvirus polycistronic mRNA
- PMID: 24623444
- PMCID: PMC4093840
- DOI: 10.1128/JVI.00202-14
Reinitiation after translation of two upstream open reading frames (ORF) governs expression of the ORF35-37 Kaposi's sarcoma-associated herpesvirus polycistronic mRNA
Abstract
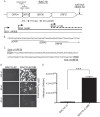
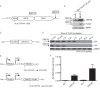
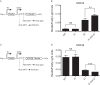
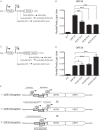
The Kaposi's sarcoma-associated herpesvirus (KSHV) ORF36 protein kinase is translated as a downstream gene from the ORF35-37 polycistronic mRNA via a unique mechanism involving short upstream open reading frames (uORFs) located in the 5' untranslated region. Here, we confirm that ORF35-37 is functionally dicistronic during infection and demonstrate that mutation of the dominant uORF restricts KSHV replication. Leaky scanning past the uORFs facilitates ORF35 expression, while a reinitiation mechanism after translation of the uORFs enables ORF36 expression.
Figures
References
-
- Martin DF, Kuppermann BD, Wolitz RA, Palestine AG, Li H, Robinson CA. 1999. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N. Engl. J. Med. 340:1063–1070 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA160556/CA/NCI NIH HHS/United States
- R01 CA082057/CA/NCI NIH HHS/United States
- CA31363/CA/NCI NIH HHS/United States
- R01 CA115284/CA/NCI NIH HHS/United States
- R01 AI073099/AI/NIAID NIH HHS/United States
- R01 CA136367/CA/NCI NIH HHS/United States
- R01 CA031363/CA/NCI NIH HHS/United States
- CA160556/CA/NCI NIH HHS/United States
- AI073099/AI/NIAID NIH HHS/United States
- R01 DE023926/DE/NIDCR NIH HHS/United States
- DE023926/DE/NIDCR NIH HHS/United States
- CA082057/CA/NCI NIH HHS/United States
- CA115284/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

