A novel secreted metalloprotease (CD2830) from Clostridium difficile cleaves specific proline sequences in LPXTG cell surface proteins
- PMID: 24623589
- PMCID: PMC4014281
- DOI: 10.1074/mcp.M113.034728
A novel secreted metalloprotease (CD2830) from Clostridium difficile cleaves specific proline sequences in LPXTG cell surface proteins
Abstract
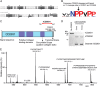
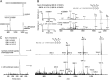
Bacterial secreted proteins constitute a biologically important subset of proteins involved in key processes related to infection such as adhesion, colonization, and dissemination. Bacterial extracellular proteases, in particular, have attracted considerable attention, as they have been shown to be indispensable for bacterial virulence. Here, we analyzed the extracellular subproteome of Clostridium difficile and identified a hypothetical protein, CD2830, as a novel secreted metalloprotease. Following the identification of a CD2830 cleavage site in human HSP90β, a series of synthetic peptide substrates was used to identify the favorable CD2830 cleavage motif. This motif was characterized by a high prevalence of proline residues. Intriguingly, CD2830 has a preference for cleaving Pro-Pro bonds, unique among all hitherto described proteases. Strikingly, within the C. difficile proteome two putative adhesion molecules, CD2831 and CD3246, were identified that contain multiple CD2830 cleavage sites (13 in total). We subsequently found that CD2830 efficiently cleaves CD2831 between two prolines at all predicted cleavage sites. Moreover, native CD2830, secreted by live cells, cleaves endogenous CD2831 and CD3246. These findings highlight CD2830 as a highly specific endoproteinase with a preference for proline residues surrounding the scissile bond. Moreover, the efficient cleavage of two putative surface adhesion proteins points to a possible role of CD2830 in the regulation of C. difficile adhesion.
Figures




References
-
- Rupnik M., Wilcox M. H., Gerding D. N. (2009) Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat. Rev. Microbiol. 7, 526–536 - PubMed
-
- Kuehne S. A., Cartman S. T., Heap J. T., Kelly M. L., Cockayne A., Minton N. P. (2010) The role of toxin A and toxin B in Clostridium difficile infection. Nature 467, 711–713 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

