Spinal glutamatergic neurons defined by EphA4 signaling are essential components of normal locomotor circuits
- PMID: 24623763
- PMCID: PMC6705281
- DOI: 10.1523/JNEUROSCI.4992-13.2014
Spinal glutamatergic neurons defined by EphA4 signaling are essential components of normal locomotor circuits
Abstract
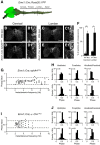
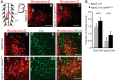
EphA4 signaling is essential for the spatiotemporal organization of neuronal circuit formation. In mice, deletion of this signaling pathway causes aberrant midline crossing of axons from both brain and spinal neurons and the complete knock-outs (KOs) exhibit a pronounced change in motor behavior, where alternating gaits are replaced by a rabbit-like hopping gait. The neuronal mechanism that is responsible for the gait switch in these KO mice is not known. Here, using intersectional genetics, we demonstrate that a spinal cord-specific deletion of EphA4 signaling is sufficient to generate the overground hopping gait. In contrast, selective deletion of EphA4 signaling in forebrain neurons, including the corticospinal tract neurons, did not result in a change in locomotor pattern. The gait switch was attributed to the loss of EphA4 signaling in excitatory Vglut2+ neurons, which is accompanied by an increased midline crossing of Vglut2+ neurons in the ventral spinal cord. Our findings functionally define spinal EphA4 signaling in excitatory Vglut2+ neurons as required for proper organization of the spinal locomotor circuitry, and place these cells as essential components of the mammalian locomotor network.
Keywords: EphA4; central pattern generator; locomotion; spinal cord; α-chimaerin.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
