Excitatory synaptic inputs to mouse on-off direction-selective retinal ganglion cells lack direction tuning
- PMID: 24623775
- PMCID: PMC3951696
- DOI: 10.1523/JNEUROSCI.5017-13.2014
Excitatory synaptic inputs to mouse on-off direction-selective retinal ganglion cells lack direction tuning
Abstract
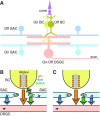
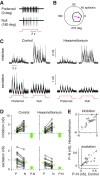
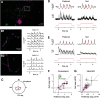
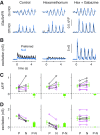
Direction selectivity represents a fundamental visual computation. In mammalian retina, On-Off direction-selective ganglion cells (DSGCs) respond strongly to motion in a preferred direction and weakly to motion in the opposite, null direction. Electrical recordings suggested three direction-selective (DS) synaptic mechanisms: DS GABA release during null-direction motion from starburst amacrine cells (SACs) and DS acetylcholine and glutamate release during preferred direction motion from SACs and bipolar cells. However, evidence for DS acetylcholine and glutamate release has been inconsistent and at least one bipolar cell type that contacts another DSGC (On-type) lacks DS release. Here, whole-cell recordings in mouse retina showed that cholinergic input to On-Off DSGCs lacked DS, whereas the remaining (glutamatergic) input showed apparent DS. Fluorescence measurements with the glutamate biosensor intensity-based glutamate-sensing fluorescent reporter (iGluSnFR) conditionally expressed in On-Off DSGCs showed that glutamate release in both On- and Off-layer dendrites lacked DS, whereas simultaneously recorded excitatory currents showed apparent DS. With GABA-A receptors blocked, both iGluSnFR signals and excitatory currents lacked DS. Our measurements rule out DS release from bipolar cells onto On-Off DSGCs and support a theoretical model suggesting that apparent DS excitation in voltage-clamp recordings results from inadequate voltage control of DSGC dendrites during null-direction inhibition. SAC GABA release is the apparent sole source of DS input onto On-Off DSGCs.
Keywords: direction selectivity; glutamate sensor; mouse retina; retinal ganglion cell; synaptic mechanism; two-photon imaging.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
