Oxidative stress and adrenocortical insufficiency
- PMID: 24623797
- PMCID: PMC4045218
- DOI: 10.1530/JOE-13-0346
Oxidative stress and adrenocortical insufficiency
Abstract
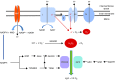
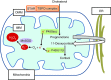
Maintenance of redox balance is essential for normal cellular functions. Any perturbation in this balance due to increased reactive oxygen species (ROS) leads to oxidative stress and may lead to cell dysfunction/damage/death. Mitochondria are responsible for the majority of cellular ROS production secondary to electron leakage as a consequence of respiration. Furthermore, electron leakage by the cytochrome P450 enzymes may render steroidogenic tissues acutely vulnerable to redox imbalance. The adrenal cortex, in particular, is well supplied with both enzymatic (glutathione peroxidases and peroxiredoxins) and non-enzymatic (vitamins A, C and E) antioxidants to cope with this increased production of ROS due to steroidogenesis. Nonetheless oxidative stress is implicated in several potentially lethal adrenal disorders including X-linked adrenoleukodystrophy, triple A syndrome and most recently familial glucocorticoid deficiency. The finding of mutations in antioxidant defence genes in the latter two conditions highlights how disturbances in redox homeostasis may have an effect on adrenal steroidogenesis.
Keywords: adrenal insufficiency; oxidative stress; reactive oxygen species; steroidogenesis.
© 2014 The authors.
Figures

References
-
- Baker BY, Lin L, Kim CJ, Raza J, Smith CP, Miller WL, Achermann JC. Nonclassic congenital lipoid adrenal hyperplasia: a new disorder of the steroidogenic acute regulatory protein with very late presentation and normal male genitalia. Journal of Clinical Endocrinology and Metabolism. 2006;91:4781–4785. doi: 10.1210/jc.2006-1565. - DOI - PMC - PubMed
-
- Behne D, Hofer-Bosse T. Effects of a low selenium status on the distribution and retention of selenium in the rat. Journal of Nutrition. 1984;114:1289–1296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

