Impact of appetitive and aversive outcomes on brain responses: linking the animal and human literatures
- PMID: 24624062
- PMCID: PMC3941203
- DOI: 10.3389/fnsys.2014.00024
Impact of appetitive and aversive outcomes on brain responses: linking the animal and human literatures
Abstract
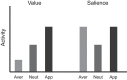
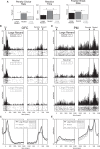
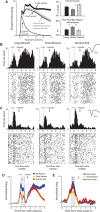
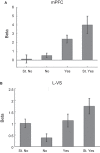
Decision-making is motivated by the possibility of obtaining reward and/or avoiding punishment. Though many have investigated behavior associated with appetitive or aversive outcomes, few have examined behaviors that rely on both. Fewer still have addressed questions related to how anticipated appetitive and aversive outcomes interact to alter neural signals related to expected value, motivation, and salience. Here we review recent rodent, monkey, and human research that address these issues. Further development of this area will be fundamental to understanding the etiology behind human psychiatric diseases and cultivating more effective treatments.
Keywords: appetitive and aversive outcomes; neural encoding; reward processing; salience; value encoding.
Figures
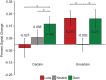
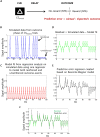
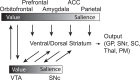
References
-
- Adolphs R., Tranel D. (2000). “Emotion recognition and the human amygdala,” in The Amygdala: A Functional Anlysis, ed Aggleton J. P. (New York: Oxford University Press; ), 587–630
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

