Role of advanced glycation end products in cellular signaling
- PMID: 24624331
- PMCID: PMC3949097
- DOI: 10.1016/j.redox.2013.12.016
Role of advanced glycation end products in cellular signaling
Abstract
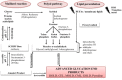
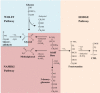
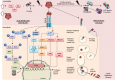
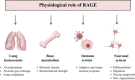
Improvements in health care and lifestyle have led to an elevated lifespan and increased focus on age-associated diseases, such as neurodegeneration, cardiovascular disease, frailty and arteriosclerosis. In all these chronic diseases protein, lipid or nucleic acid modifications are involved, including cross-linked and non-degradable aggregates, such as advanced glycation end products (AGEs). Formation of endogenous or uptake of dietary AGEs can lead to further protein modifications and activation of several inflammatory signaling pathways. This review will give an overview of the most prominent AGE-mediated signaling cascades, AGE receptor interactions, prevention of AGE formation and the impact of AGEs during pathophysiological processes.
Keywords: ADAMST, a disintegrin and metalloproteinase with a thrombospondin type 1 motif; AGE, advanced glycation end products; AGE-receptors; Advanced glycation end products; Age-associated diseases; Aggregates; Aging; E, from embryonic day; EGFR, epidermal growth factor receptor; ERK, extracellular-signal regulated kinase; F3NK, fructosamine 3-phosphokinase; FKHRL1, forkhead transcription factor; HDL, high density lipoprotein; HMGB1, high-mobility-group-protein B1; HNE, 4-hydroxy-trans-2-nonenal; Jak1/2, Janus kinase 1/2; LDL, low density lipoprotein; MDA, malondialdehyde; MEKK, mitogen-activated protein/ERK kinase kinases; MnSOD, manganese superoxide dismutase; NF-κB; Nf-κB, nuclear factor-light-chain-enhancer of activated B; Oxidative stress; PIK3, phosphoinositol 3 kinase; RAGE; RAGE, receptor of AGEs; RCC, reactive carbonyl compounds; Reactive carbonyl compounds; S100B, S100 calcium binding protein B; SIRt1, NAD+-dependent deacetylase and survival factor 1; SR-A, scavenger receptor class A; Signaling; Stat 1/2, signal transducers and activators of transcription 1/2; VSMC, vascular smooth muscle cells.
Figures


References
-
- Vaupel J.W., Kistowski K.G.V. The remarkable rise in life expectancy and how it will affect medicine. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz. 2005;48:586–592. - PubMed
-
- Oeppen J., Vaupel J.W. Demography. Broken limits to life expectancy. Science. 2002;296:1029–1031. - PubMed
-
- Singh R., Barden A., Mori T., Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. - PubMed
-
- Farouque H.M., O’Brien R.C., Meredith I.T. Diabetes mellitus and coronary heart disease—from prevention to intervention: part I. Aust. N. Z. J. Med. 2000;30:351–359. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

