Evaluation of novel oral vaccine candidates and validation of a caprine model of Johne's disease
- PMID: 24624365
- PMCID: PMC3941644
- DOI: 10.3389/fcimb.2014.00026
Evaluation of novel oral vaccine candidates and validation of a caprine model of Johne's disease
Abstract
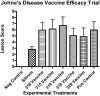
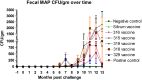
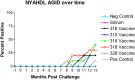
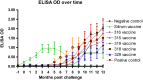
Johne's disease (JD) caused by Mycobacterium avium subspecies paratuberculosis (MAP) is a major threat to the dairy industry and possibly some cases of Crohn's disease in humans. A MAP vaccine that reduced of clinical disease and/or reduced fecal shedding would aid in the control of JD. The objectives of this study were (1) to evaluate the efficacy of 5 attenuated strains of MAP as vaccine candidates compared to a commercial control vaccine using the protocol proposed by the Johne's Disease Integrated Program (JDIP) Animal Model Standardization Committee (AMSC), and (2) to validate the AMSC Johne's disease goat challenge model. Eighty goat kids were vaccinated orally twice at 8 and 10 weeks of age with an experimental vaccine or once subcutaneously at 8 weeks with Silirum® (Zoetis), or a sham control oral vaccine at 8 and 10 weeks. Kids were challenged orally with a total of approximately 1.44 × 10(9) CFU divided in two consecutive daily doses using MAP ATCC-700535 (K10-like bovine isolate). All kids were necropsied at 13 months post-challenge. Results indicated that the AMSC goat challenge model is a highly efficient and valid model for JD challenge studies. None of the experimental or control vaccines evaluated prevented MAP infection or eliminated fecal shedding, although the 329 vaccine lowered the incidence of infection, fecal shedding, tissue colonization and reduced lesion scores, but less than the control vaccine. Based on our results the relative performance ranking of the experimental live-attenuated vaccines evaluated, the 329 vaccine was the best performer, followed by the 318 vaccine, then 316 vaccine, 315 vaccine and finally the 319 vaccine was the worst performer. The subcutaneously injected control vaccine outperformed the orally-delivered mutant vaccine candidates. Two vaccines (329 and 318) do reduce presence of JD gross and microscopic lesions, slow progression of disease, and one vaccine (329) reduced fecal shedding and tissue colonization.
Keywords: Mycobacterium avium subsp paratuberculosis; diagnostic tests; goats; mutant vaccines; vaccine efficacy.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

