Cell-penetrating peptide (CPP)-conjugated proteins is an efficient tool for manipulation of human mesenchymal stromal cells
- PMID: 24625570
- PMCID: PMC3953728
- DOI: 10.1038/srep04378
Cell-penetrating peptide (CPP)-conjugated proteins is an efficient tool for manipulation of human mesenchymal stromal cells
Abstract
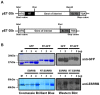
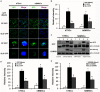
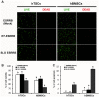
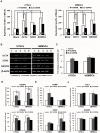
Delivery of proteins has been regarded as the safest and most useful application in therapeutic application of stem cells, because proteins can regulate gene expression transiently without any genomic alteration. However, it is difficult to accurately measure efficiency or quantity of intracellular protein uptake. Here, we performed a comparison study of cell-penetrating peptide (CPP)-conjugated protein delivery system using seven arginine and Streptolysin O (SLO)-mediated system. To compare CPP- and SLO-mediated protein delivery systems, we used GFP and ESRRB protein, which is known to regulate pluripotency-related genes, for delivery into human bone marrow stromal cells (hBMSCs) and human testicular stromal cells (hTSCs). We found that CPP-conjugated protein delivery was more efficient, lower cytotoxicity, and higher biological activity than SLO-mediated protein delivery system. These results suggest that delivery of CPP-conjugated proteins is an efficient tool for introducing biologically active proteins into cells and may have important implications in clinical cell-based therapy.
Figures
References
-
- O'Malley J., Woltjen K. & Kaji K. New strategies to generate induced pluripotent stem cells. Curr Opin Biotechnol 20, 516–21 (2009). - PubMed
-
- Derossi D., Joliot A. H., Chassaing G. & Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem 269, 10444–50 (1994). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

