Long term streptomycin toxicity in the treatment of Buruli Ulcer: follow-up of participants in the BURULICO drug trial
- PMID: 24625583
- PMCID: PMC3953024
- DOI: 10.1371/journal.pntd.0002739
Long term streptomycin toxicity in the treatment of Buruli Ulcer: follow-up of participants in the BURULICO drug trial
Abstract
Background: Buruli Ulcer (BU) is a tropical infectious skin disease that is currently treated with 8 weeks of intramuscular streptomycin and oral rifampicin. As prolonged streptomycin administration can cause both oto- and nephrotoxicity, we evaluated its long term toxicity by following-up former BU patients that had received either 4 or 8 weeks of streptomycin in addition to other drugs between 2006 and 2008, in the context of a randomized controlled trial.
Methods: Former patients were retrieved in 2012, and oto- and nephrotoxicity were determined by audiometry and serum creatinine levels. Data were compared with baseline and week 8 measurements during the drug trial.
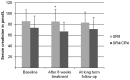
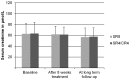
Results: Of the total of 151 former patients, 127 (84%) were retrieved. Ototoxicity was present in 29% of adults and 25% of children. Adults in the 8 week streptomycin group had significantly higher hearing thresholds in all frequencies at long term follow-up, and these differences were most prominent in the high frequencies. In children, no differences between the two treatment arms were found. Nephrotoxicity that had been detected in 14% of adults and in 13% of children during treatment, was present in only 2.4% of patients at long term follow-up.
Conclusions: Prolonged streptomycin administration in the adult study subjects caused significant persistent hearing loss, especially in the high frequency range. Nephrotoxicity was also present in both adults and children but appeared to be transient. Streptomycin should be given with caution especially in patients aged 16 or older, and in individuals with concurrent risks for renal dysfunction or hearing loss.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Stienstra Y, van Roest MH, van Wezel MJ, Wiersma IC, Hospers IC, et al. (2005) Factors associated with functional limitations and subsequent employment or schooling in buruli ulcer patients. Trop Med Int Health 10 (12) 1251–1257. - PubMed
-
- Wansbrough-Jones M, Phillips R (2006) Buruli ulcer: Emerging from obscurity. Lancet 367 (9525) 1849–1858. - PubMed
-
- [Anonymous]. (2012) WHO fact sheet on buruli ulcer. 2013(Jan 30).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

