Surgical sutures filled with adipose-derived stem cells promote wound healing
- PMID: 24625821
- PMCID: PMC3953386
- DOI: 10.1371/journal.pone.0091169
Surgical sutures filled with adipose-derived stem cells promote wound healing
Abstract
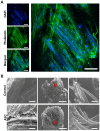
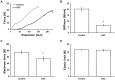
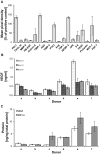
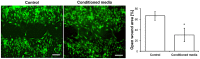
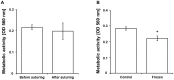
Delayed wound healing and scar formation are among the most frequent complications after surgical interventions. Although biodegradable surgical sutures present an excellent drug delivery opportunity, their primary function is tissue fixation. Mesenchymal stem cells (MSC) act as trophic mediators and are successful in activating biomaterials. Here biodegradable sutures were filled with adipose-derived mesenchymal stem cells (ASC) to provide a pro-regenerative environment at the injured site. Results showed that after filling, ASCs attach to the suture material, distribute equally throughout the filaments, and remain viable in the suture. Among a broad panel of cytokines, cell-filled sutures constantly release vascular endothelial growth factor to supernatants. Such conditioned media was evaluated in an in vitro wound healing assay and showed a significant decrease in the open wound area compared to controls. After suturing in an ex vivo wound model, cells remained in the suture and maintained their metabolic activity. Furthermore, cell-filled sutures can be cryopreserved without losing their viability. This study presents an innovative approach to equip surgical sutures with pro-regenerative features and allows the treatment and fixation of wounds in one step, therefore representing a promising tool to promote wound healing after injury.
Conflict of interest statement
Figures


References
-
- Jones E, Yang X (2011) Mesenchymal stem cells and bone regeneration: current status. Injury 42: 562–568. - PubMed
-
- Li H, Fu X (2012) Mechanisms of action of mesenchymal stem cells in cutaneous wound repair and regeneration. Cell Tissue Res 348: 371–377. - PubMed
-
- Brower J, Blumberg S, Carroll E, Pastar I, Brem H, et al. (2011) Mesenchymal stem cell therapy and delivery systems in nonhealing wounds. Adv Skin Wound Care 24: 524–532; quiz 533–524. - PubMed
-
- Choi KH, Choi BH, Park SR, Kim BJ, Min BH (2010) The chondrogenic differentiation of mesenchymal stem cells on an extracellular matrix scaffold derived from porcine chondrocytes. Biomaterials 31: 5355–5365. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

