Ubiquitin-like (UBX)-domain-containing protein, UBXN2A, promotes cell death by interfering with the p53-Mortalin interactions in colon cancer cells
- PMID: 24625977
- PMCID: PMC3973214
- DOI: 10.1038/cddis.2014.100
Ubiquitin-like (UBX)-domain-containing protein, UBXN2A, promotes cell death by interfering with the p53-Mortalin interactions in colon cancer cells
Abstract
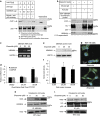
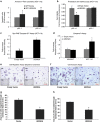
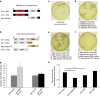
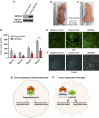
Mortalin (mot-2) induces inactivation of the tumor suppressor p53's transcriptional and apoptotic functions by cytoplasmic sequestration of p53 in select cancers. The mot-2-dependent cytoprotective function enables cancer cells to support malignant transformation. Abrogating the p53-mot-2 interaction can control or slow down the growth of cancer cells. In this study, we report the discovery of a ubiquitin-like (UBX)-domain-containing protein, UBXN2A, which binds to mot-2 and consequently inhibits the binding between mot-2 and p53. Genetic analysis showed that UBXN2A binds to mot-2's substrate binding domain, and it partly overlaps p53's binding site indicating UBXN2A and p53 likely bind to mot-2 competitively. By binding to mot-2, UBXN2A releases p53 from cytosolic sequestration, rescuing the tumor suppressor functions of p53. Biochemical analysis and functional assays showed that the overexpression of UBXN2A and the functional consequences of unsequestered p53 trigger p53-dependent apoptosis. Cells expressing shRNA against UBXN2A showed the opposite effect of that seen with UBXN2A overexpression. The expression of UBXN2A and its apoptotic effects were not observed in normal colonic epithelial cells and p53-/- colon cancer cells. Finally, significant reduction in tumor volume in a xenograft mouse model in response to UBXN2A expression was verified in vivo. Our results introduce UBXN2A as a home defense response protein, which can reconstitute inactive p53-dependent apoptotic pathways. Inhibition of mot-2-p53 interaction by UBXN2A is an attractive therapeutic strategy in mot-2-elevated tumors.
Figures




References
-
- Dundas SR, Lawrie LC, Rooney PH, Murray GI. Mortalin is over-expressed by colorectal adenocarcinomas and correlates with poor survival. J Pathol. 2005;205:74–81. - PubMed
-
- Takano S, Wadhwa R, Yoshii Y, Nose T, Kaul SC, Mitsui Y. Elevated levels of mortalin expression in human brain tumors. Exp Cell Res. 1997;237:38–45. - PubMed
-
- Wadhwa R, Takano S, Kaur K, Deocaris CC, Pereira-Smith OM, Reddel RR, et al. Upregulation of mortalin/mthsp70/Grp75 contributes to human carcinogenesis. Int J Cancer. 2006;118:2973–2980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

