Inflammasome sensor NLRP1 controls rat macrophage susceptibility to Toxoplasma gondii
- PMID: 24626226
- PMCID: PMC3953412
- DOI: 10.1371/journal.ppat.1003927
Inflammasome sensor NLRP1 controls rat macrophage susceptibility to Toxoplasma gondii
Abstract
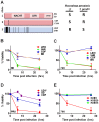
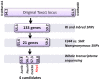
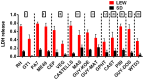
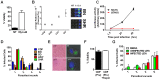
Toxoplasma gondii is an intracellular parasite that infects a wide range of warm-blooded species. Rats vary in their susceptibility to this parasite. The Toxo1 locus conferring Toxoplasma resistance in rats was previously mapped to a region of chromosome 10 containing Nlrp1. This gene encodes an inflammasome sensor controlling macrophage sensitivity to anthrax lethal toxin (LT) induced rapid cell death (pyroptosis). We show here that rat strain differences in Toxoplasma infected macrophage sensitivity to pyroptosis, IL-1β/IL-18 processing, and inhibition of parasite proliferation are perfectly correlated with NLRP1 sequence, while inversely correlated with sensitivity to anthrax LT-induced cell death. Using recombinant inbred rats, SNP analyses and whole transcriptome gene expression studies, we narrowed the candidate genes for control of Toxoplasma-mediated rat macrophage pyroptosis to four genes, one of which was Nlrp1. Knockdown of Nlrp1 in pyroptosis-sensitive macrophages resulted in higher parasite replication and protection from cell death. Reciprocally, overexpression of the NLRP1 variant from Toxoplasma-sensitive macrophages in pyroptosis-resistant cells led to sensitization of these resistant macrophages. Our findings reveal Toxoplasma as a novel activator of the NLRP1 inflammasome in rat macrophages.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Pravenec M, Gauguier D, Schott JJ, Buard J, Kren V, et al. (1996) A genetic linkage map of the rat derived from recombinant inbred strains. Mamm Genome 7: 117–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

