Omega-3 fatty acid supplementation during pregnancy and respiratory symptoms in children
- PMID: 24626819
- PMCID: PMC4122276
- DOI: 10.1378/chest.13-1432
Omega-3 fatty acid supplementation during pregnancy and respiratory symptoms in children
Abstract
Background: Prenatal consumption of omega-3 fatty acids can act as an adjuvant in the development of the immune system and affect the inflammatory response of neonates.
Methods: We conducted a double-blind, randomized, placebo-controlled trial in Cuernavaca, Mexico. We randomly assigned 1,094 pregnant women (18-35 years of age) to receive 400 mg/d of algal docosahexaenoic acid (DHA) or placebo from 18 to 22 weeks of gestation through delivery. Birth outcomes and respiratory symptoms information until 18 months were available for 869 mother-child pairs. Questionnaires were administered, and maternal blood samples were obtained at baseline. Maternal atopy was based on specific IgE levels. During follow-up, information on infants' respiratory symptoms was collected through questionnaires administered at 1, 3, 6, 9, 12, and 18 months of age. Negative binomial regression models were used to evaluate the effect of supplementation on respiratory symptoms in infants.
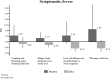
Results: Among infants of atopic mothers, a statistically significant protective effect of DHA treatment was observed on phlegm with nasal discharge or nasal congestion (0.78; 95% CI, 0.60-1.02) and fever with phlegm and nasal discharge or nasal congestion (0.53; 95% CI, 0.29-0.99), adjusting for potential confounders.
Conclusions: Our results support the hypothesis that DHA supplementation during pregnancy may decrease the incidence of respiratory symptoms in children with a history of maternal atopy.
Trial registry: ClinicalTrials.gov; No.: NCT00646360; URL: www.clinicaltrials.gov.
Figures


References
-
- Rabinovich GA. Inmunopatología Molecular Nuevas Fronteras de la Medicina. Un Nexo Entre la Investigación Biomédica y la Práctica Clínica. Buenos Aires, Argentina: Editorial Panamericana; 2004
-
- Secretaria de Salud. Anuario Estadístico 2009. Presupuesto, daños a la salud, morbilidad y mortalidad. Cuernavaca, Morelos: Secretaria de Salud, Servicios de Salud de Morelos, 2009. http://www.ssm.gob.mx/portal/page/sist_info_salud/anuarios/2009/A4_2009_.... Accessed November 18, 2002
-
- Fieselmann JF, Hal BR, Gottrand F. Enfermedades respiratorias. In: Parslow TG, Stites DP, Terr AL, Imboden JB. eds. Inmunogía Básica y Clínica. México, D. F.: El Manual Moderno; 2003:663-678
-
- Gottrand F. Long-chain polyunsaturated fatty acids influence the immune system of infants. J Nutr. 2008;138(9):1807S-1812S - PubMed
-
- Blümer N, Renz H. Consumption of omega3-fatty acids during perinatal life: role in immuno-modulation and allergy prevention. J Perinat Med. 2007;35(suppl 1):S12-S18 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

