Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise
- PMID: 24627275
- PMCID: PMC3982888
- DOI: 10.1093/jnci/dju036
Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise
Abstract
Background: Previous studies have hypothesized that tumor blood flow may be elevated or reduced during exercise, which could impact the tumor microenvironment. However, to date technical limitations have precluded the measurement of tumor blood flow during exercise. Using an orthotopic preclinical model of prostate cancer, we tested the hypotheses that during exercise tumors would experience 1) diminished vascular resistance, 2) augmented blood flow, 3) increased numbers of perfused vessels, and 4) decreased tissue hypoxia and, furthermore, that the increased perfusion would be associated with diminished vasoconstriction in prostate tumor arterioles.
Methods: Dunning R-3327 MatLyLu tumor cells were injected into the ventral prostate of male Copenhagen rats aged 4 to 6 months randomly assigned to tumor-bearing (n = 42) or vehicle control (n = 14) groups. Prostate tumor blood flow, vascular resistance, patent vessel number, and hypoxia were measured in vivo in conscious rats at rest and during treadmill exercise, and vasoconstrictor responsiveness of resistance arterioles was investigated in vitro.
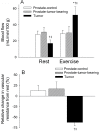
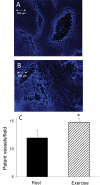
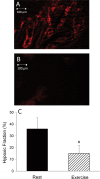
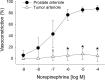
Results: During exercise there was a statistically significant increase in tumor blood flow (approximately 200%) and number of patent vessels (rest mean ± standard deviation [SD] = 12.7±1.3; exercise mean ± SD = 14.3±0.6 vessels/field; Student t test two-sided P = .02) and decreased hypoxia compared with measurements made at rest. In tumor arterioles, the maximal constriction elicited by norepinephrine was blunted by approximately 95% vs control prostate vessels.
Conclusions: During exercise there is enhanced tumor perfusion and diminished tumor hypoxia due, in part, to a diminished vasoconstriction. The clinical relevance of these findings are that exercise may enhance the delivery of tumor-targeting drugs as well as attenuate the hypoxic microenvironment within a tumor and lead to a less aggressive phenotype.
Figures

Comment in
-
Therapeutic properties of aerobic training after a cancer diagnosis: more than a one-trick pony?J Natl Cancer Inst. 2014 Apr;106(4):dju042. doi: 10.1093/jnci/dju042. Epub 2014 Mar 13. J Natl Cancer Inst. 2014. PMID: 24627274 Free PMC article. No abstract available.
References
-
- Mereles D, Ehlken N, Kreuscher S, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation. 2006;114(14):1482–1489 - PubMed
-
- National Cancer Institute Physical Activity and Cancer. http://www.cancer.gov/cancertopics/factsheet/prevention/physicalactivity Accessed February 12, 2014
-
- Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274 - PubMed
-
- Musch TI, Friedman DB, Pitetti KH, et al. Regional distribution of blood flow of dogs during graded dynamic exercise. J Appl Physiol. 1987;63(6):2269–2277 - PubMed
-
- Rowell L. Human Cardiovascular Control. Oxford: Oxford University Press; 1993
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

