Glycosylphosphatidylinositol anchoring directs the assembly of Sup35NM protein into non-fibrillar, membrane-bound aggregates
- PMID: 24627481
- PMCID: PMC4007424
- DOI: 10.1074/jbc.M114.556639
Glycosylphosphatidylinositol anchoring directs the assembly of Sup35NM protein into non-fibrillar, membrane-bound aggregates
Abstract
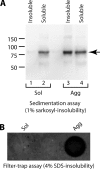
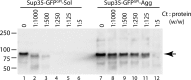
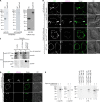
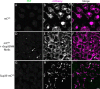
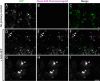
In prion-infected hosts, PrPSc usually accumulates as non-fibrillar, membrane-bound aggregates. Glycosylphosphatidylinositol (GPI) anchor-directed membrane association appears to be an important factor controlling the biophysical properties of PrPSc aggregates. To determine whether GPI anchoring can similarly modulate the assembly of other amyloid-forming proteins, neuronal cell lines were generated that expressed a GPI-anchored form of a model amyloidogenic protein, the NM domain of the yeast prion protein Sup35 (Sup35(GPI)). We recently reported that GPI anchoring facilitated the induction of Sup35(GPI) prions in this system. Here, we report the ultrastructural characterization of self-propagating Sup35(GPI) aggregates of either spontaneous or induced origin. Like membrane-bound PrPSc, Sup35(GPI) aggregates resisted release from cells treated with phosphatidylinositol-specific phospholipase C. Sup35(GPI) aggregates of spontaneous origin were detergent-insoluble, protease-resistant, and self-propagating, in a manner similar to that reported for recombinant Sup35NM amyloid fibrils and induced Sup35(GPI) aggregates. However, GPI-anchored Sup35 aggregates were not stained with amyloid-binding dyes, such as Thioflavin T. This was consistent with ultrastructural analyses, which showed that the aggregates corresponded to dense cell surface accumulations of membrane vesicle-like structures and were not fibrillar. Together, these results showed that GPI anchoring directs the assembly of Sup35NM into non-fibrillar, membrane-bound aggregates that resemble PrPSc, raising the possibility that GPI anchor-dependent modulation of protein aggregation might occur with other amyloidogenic proteins. This may contribute to differences in pathogenesis and pathology between prion diseases, which uniquely involve aggregation of a GPI-anchored protein, versus other protein misfolding diseases.
Keywords: Amyloid; Electron Microscopy (EM); Glycosylphosphatidylinositol Anchors; Prions; Protein Aggregation; Protein Misfolding; Protein Self-assembly.
Figures








References
-
- Caughey B. W., Dong A., Bhat K. S., Ernst D., Hayes S. F., Caughey W. S. (1991) Secondary structure analysis of the scrapie-associated protein PrP 27–30 in water by infrared spectroscopy. Biochemistry 30, 7672–7680 - PubMed
-
- Prusiner S. B., Groth D. F., Bolton D. C., Kent S. B., Hood L. E. (1984) Purification and structural studies of a major scrapie prion protein. Cell 38, 127–134 - PubMed
-
- Jarrett J. T., Lansbury P. T., Jr. (1993) Seeding “One-Dimensional Crystallization” of Amyloid: a Pathogenic Mechanism in Alzheimer's disease and scrapie? Cell 73, 1055–1058 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

