Yeast AMP-activated protein kinase monitors glucose concentration changes and absolute glucose levels
- PMID: 24627493
- PMCID: PMC4007474
- DOI: 10.1074/jbc.M114.547976
Yeast AMP-activated protein kinase monitors glucose concentration changes and absolute glucose levels
Abstract
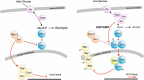
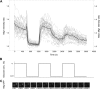
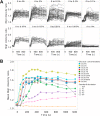
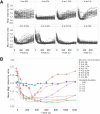
Analysis of the time-dependent behavior of a signaling system can provide insight into its dynamic properties. We employed the nucleocytoplasmic shuttling of the transcriptional repressor Mig1 as readout to characterize Snf1-Mig1 dynamics in single yeast cells. Mig1 binds to promoters of target genes and mediates glucose repression. Mig1 is predominantly located in the nucleus when glucose is abundant. Upon glucose depletion, Mig1 is phosphorylated by the yeast AMP-activated kinase Snf1 and exported into the cytoplasm. We used a three-channel microfluidic device to establish a high degree of control over the glucose concentration exposed to cells. Following regimes of glucose up- and downshifts, we observed a very rapid response reaching a new steady state within less than 1 min, different glucose threshold concentrations depending on glucose up- or downshifts, a graded profile with increased cell-to-cell variation at threshold glucose concentrations, and biphasic behavior with a transient translocation of Mig1 upon the shift from high to intermediate glucose concentrations. Fluorescence loss in photobleaching and fluorescence recovery after photobleaching data demonstrate that Mig1 shuttles constantly between the nucleus and cytoplasm, although with different rates, depending on the presence of glucose. Taken together, our data suggest that the Snf1-Mig1 system has the ability to monitor glucose concentration changes as well as absolute glucose levels. The sensitivity over a wide range of glucose levels and different glucose concentration-dependent response profiles are likely determined by the close integration of signaling with the metabolism and may provide for a highly flexible and fast adaptation to an altered nutritional status.
Keywords: AMP-activated Kinase (AMPK); Dynamic Control; Glucose Metabolism; Nuclear Translocation; Signal Transduction; Yeast Physiology.
Figures



References
-
- Pelet S., Rudolf F., Nadal-Ribelles M., de Nadal E., Posas F., Peter M. (2011) Transient activation of the HOG MAPK pathway regulates bimodal gene expression. Science 332, 732–735 - PubMed
-
- Geijer C., Medrala-Klein D., Petelenz-Kurdziel E., Ericsson A., Smedh M., Andersson M., Goksör M., Nadal-Ribelles M., Posas F., Krantz M., Nordlander B., Hohmann S. (2013) Initiation of the transcriptional response to hyperosmotic shock correlates with the potential for volume recovery. FEBS J. 280, 3854–3867 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

