Nicotine patches in pregnant smokers: randomised, placebo controlled, multicentre trial of efficacy
- PMID: 24627552
- PMCID: PMC3950302
- DOI: 10.1136/bmj.g1622
Nicotine patches in pregnant smokers: randomised, placebo controlled, multicentre trial of efficacy
Abstract
Objective: To determine the efficacy of 16 hour nicotine patches among pregnant smokers, with the dose individually adjusted according to saliva cotinine levels (potential range 10-30 mg/day).
Design: Randomised, double blind, placebo controlled, parallel group, multicentre trial (Study of Nicotine Patch in Pregnancy, SNIPP) between October 2007 and January 2013.
Setting: 23 maternity wards in France.
Participants: 476 pregnant smokers aged more than 18 years and between 12 and 20 weeks' gestation, who smoked at least five cigarettes a day. After exclusions, 402 women were randomised: 203 to nicotine patches and 199 to placebo patches. Data were available on 192 live births in each group.
Interventions: Nicotine and identical placebo patches were administered from quit day up to the time of delivery. Doses were adjusted to saliva cotinine levels when smoking to yield a substitution rate of 100%. Participants were assessed monthly and received behavioural smoking cessation support.
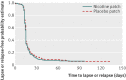
Main outcome measures: The primary outcomes were complete abstinence (self report confirmed by carbon monoxide level in expired air ≤ 8 ppm) from quit date to delivery, and birth weight. The secondary outcomes were point prevalence of abstinence, time to lapse (a few puffs) or relapse, and delivery and birth characteristics. All data were analysed on an intention to treat basis.
Results: Complete abstinence was achieved by 5.5% (n=11) of women in the nicotine patch group and 5.1% (n=10) in the placebo patch group (odds ratio 1.08, 95% confidence interval 0.45 to 2.60). The median time to the first cigarette smoked after target quit day was 15 days in both groups (interquartile range 13-18 in the nicotine patch group, 13-20 in the placebo patch group). The point prevalence abstinence ranged from 8% to 12.5% in the nicotine patch group and 8% to 9.5% in the placebo patch group without statistically significant differences. The nicotine substitution rate did not differ from 100%, and the self reported median compliance rate was 85% (interquartile range 56-99%) in the nicotine patch group and 83% (56-95%) in the placebo patch group, assessed at 1016 visits. The mean birth weight was 3065 g (SE 44 g) in the nicotine patch group and 3015 g (SE 44 g) in the placebo patch group (P=0.41). Diastolic blood pressure was significantly higher in the nicotine patch group than in the placebo patch group. The frequency of serious adverse events was similar between the groups, although more non-serious adverse reactions, mainly of skin, occurred in the nicotine patch group.
Conclusion: The nicotine patch did not increase either smoking cessation rates or birth weights despite adjustment of nicotine dose to match levels attained when smoking, and higher than usual doses.
Trial registration: ClinicalTrials.gov NCT00507975.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures


Comment in
-
Helping pregnant smokers to quit.BMJ. 2014 Mar 11;348:g1808. doi: 10.1136/bmj.g1808. BMJ. 2014. PMID: 24620362 No abstract available.
-
ACP Journal Club. In pregnant smokers, the nicotine patch did not increase abstinence or birthweight more than placebo.Ann Intern Med. 2014 Jun 17;160(12):JC11. doi: 10.7326/0003-4819-160-12-201406170-02011. Ann Intern Med. 2014. PMID: 24935505 No abstract available.
-
[Tobacco weaning in the pregnant woman: failure of nicotine patches].Rev Prat. 2014 Jun;64(6):769. Rev Prat. 2014. PMID: 25090756 French. No abstract available.
References
-
- Surgeon General. A report of the surgeon general: how tobacco smoke causes disease, chapter 8: reproductive and developmental effects. 2010. 2013. www.surgeongeneral.gov/library/reports/tobaccosmoke/chapter8.pdf.
-
- Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: a 30-year prospective study. Am J Psychiatry 2003;160:1978-84. - PubMed
-
- Ekblad M, Gissler M, Lehtonen L, Korkeila J. Prenatal smoking exposure and the risk of psychiatric morbidity into young adulthood. Arch Gen Psychiatry 2010;67:841-9. - PubMed
-
- Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, chen Y, Cook DG, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics 2012;129:735-44. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
