Interferences from blood collection tube components on clinical chemistry assays
- PMID: 24627713
- PMCID: PMC3936985
- DOI: 10.11613/BM.2014.006
Interferences from blood collection tube components on clinical chemistry assays
Abstract
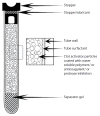
Improper design or use of blood collection devices can adversely affect the accuracy of laboratory test results. Vascular access devices, such as catheters and needles, exert shear forces during blood flow, which creates a predisposition to cell lysis. Components from blood collection tubes, such as stoppers, lubricants, surfactants, and separator gels, can leach into specimens and/or adsorb analytes from a specimen; special tube additives may also alter analyte stability. Because of these interactions with blood specimens, blood collection devices are a potential source of pre-analytical error in laboratory testing. Accurate laboratory testing requires an understanding of the complex interactions between collection devices and blood specimens. Manufacturers, vendors, and clinical laboratorians must consider the pre-analytical challenges in laboratory testing. Although other authors have described the effects of endogenous substances on clinical assay results, the effects/impact of blood collection tube additives and components have not been well systematically described or explained. This review aims to identify and describe blood collection tube additives and their components and the strategies used to minimize their effects on clinical chemistry assays.
Keywords: blood collection devices; blood collection sample tube; clinical assays; clinical chemistry; interference; pre-analytical; surfactant.
Figures

References
-
- Funderburk JV. “System for lubricating a syringe barrel”. Oct, 1995. U.S. Patent No. 5,456,940,
-
- MacNutt MJ, Sheel AW. Performance of evacuated blood collection tubes at high altitude. High Alt Med Bio. 2008;9:235–7. http://dx.doi.org/10.1089/ham.2008.1027. - DOI - PubMed
-
- Boeynaems JM, De Leener A, Dessars B, Villa-Lobos HR, Aubry JC, Cotton F, Thiry P. Evaluation of a new generation of plastic evacuated blood-collection tubes in clinical chemistry, therapeutic drug monitoring, hormone and trace metal analysis. Clin Chem Lab Med. 2004;42:67–71. http://dx.doi.org/10.1515/CCLM.2004.013. - DOI - PubMed
-
- Daves M, Trevisan D, Cemin R. Different collection tubes in cardiac biomarkers detection. J Clin Lab Anal. 2008;22:391–4. http://dx.doi.org/10.1002/jcla.20277. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

