Anacardic acid, a histone acetyltransferase inhibitor, modulates LPS-induced IL-8 expression in a human alveolar epithelial cell line A549
- PMID: 24627774
- PMCID: PMC3931454
- DOI: 10.12688/f1000research.2-78.v1
Anacardic acid, a histone acetyltransferase inhibitor, modulates LPS-induced IL-8 expression in a human alveolar epithelial cell line A549
Abstract
Objective and design: The histone acetylation processes, which are believed to play a critical role in the regulation of many inflammatory genes, are reversible and regulated by histone acetyltransferases (HATs), which promote acetylation, and histone deacetylases (HDACs), which promote deacetylation. We studied the effects of lipopolysaccharide (LPS) on histone acetylation and its role in the regulation of interleukin (IL)-8 expression.
Material: A human alveolar epithelial cell line A549 was used in vitro.
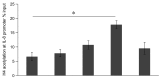
Methods: Histone H4 acetylation at the IL-8 promoter region was assessed by a chromatin immunoprecipitation (ChIP) assay. The expression and production of IL-8 were evaluated by quantitative polymerase chain reaction and specific immunoassay. Effects of a HDAC inhibitor, trichostatin A (TSA), and a HAT inhibitor, anacardic acid, were assessed.
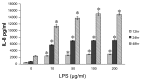
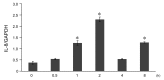
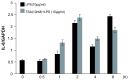
Results: Escherichia coli-derived LPS showed a dose- and time-dependent stimulatory effect on IL-8 protein production and mRNA expression in A549 cells in vitro. LPS showed a significant stimulatory effect on histone H4 acetylation at the IL-8 promoter region by ChIP assay. Pretreatment with TSA showed a dose-dependent stimulatory effect on IL-8 release from A549 cells as compared to LPS alone. Conversely, pretreatment with anacardic acid inhibited IL-8 production and expression in A549 cells.
Conclusion: These data suggest that LPS-mediated proinflammatory responses in the lungs might be modulated via changing chromatin remodeling by HAT inhibition.
Conflict of interest statement
Figures



References
-
- Gilmour PS, Rahman I, Hayashi S, et al. : Adenoviral E1A primes alveolar epithelial cells to PM(10)-induced transcription of interleukin-8. Am J Physiol Lung Cell Mol Physiol. 2001;281(3):L598–L606 - PubMed
-
- Hashimoto S, Gon Y, Takeshita I, et al. : Diesel exhaust particles activate p38 MAP kinase to produce interleukin 8 and RANTES by human bronchial epithelial cells and N-acetylcysteine attenuates p38 MAP kinase activation. Am J Respir Crit Care Med. 2000;161(1):280–5 10.1164/ajrccm.161.1.9904110 - DOI - PubMed
-
- Nakamura H, Yoshimura K, Jaffe HA, et al. : Interleukin-8 gene expression in human bronchial epithelial cells. J Biol Chem. 1991;266(29):19611–7 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

