Structure of Class B GPCRs: new horizons for drug discovery
- PMID: 24628305
- PMCID: PMC4080969
- DOI: 10.1111/bph.12689
Structure of Class B GPCRs: new horizons for drug discovery
Abstract
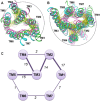
Class B GPCRs of the secretin family are important drug targets in many human diseases including diabetes, neurodegeneration, cardiovascular disease and psychiatric disorders. X-ray crystal structures for the glucagon receptor and corticotropin-releasing factor receptor 1 have now been published. In this review, we analyse the new structures and how they compare with each other and with Class A and F receptors. We also consider the differences in druggability and possible similarity in the activation mechanisms. Finally, we discuss the potential for the design of small-molecule modulators for these important targets in drug discovery. This new structural insight allows, for the first time, structure-based drug design methods to be applied to Class B GPCRs.
Keywords: CRF1 receptor; Class B; GPCR; GPCR molecular signature; HHM; druggability; glucagon receptor; smoothened receptor.
© 2014 The British Pharmacological Society.
Figures






References
-
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. - PubMed
-
- Ballesteros JA, Weinstein H, Stuart CS. Methods in Neurosciences. New York: Academic; 1995. pp. 366–428.
-
- Bissantz C, Logean A, Didier R. High-throughput modeling of human G-protein coupled receptors: amino acid sequence alignment, three-dimensional model building, and receptor library screening. J Chem Inf Comput Sci. 2004;44:1162–1176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

