Identification of candidate genes involved in coronary artery calcification by transcriptome sequencing of cell lines
- PMID: 24628908
- PMCID: PMC4003819
- DOI: 10.1186/1471-2164-15-198
Identification of candidate genes involved in coronary artery calcification by transcriptome sequencing of cell lines
Abstract
Background: Massively-parallel cDNA sequencing (RNA-Seq) is a new technique that holds great promise for cardiovascular genomics. Here, we used RNA-Seq to study the transcriptomes of matched coronary artery disease cases and controls in the ClinSeq® study, using cell lines as tissue surrogates.
Results: Lymphoblastoid cell lines (LCLs) from 16 cases and controls representing phenotypic extremes for coronary calcification were cultured and analyzed using RNA-Seq. All cell lines were then independently re-cultured and along with another set of 16 independent cases and controls, were profiled with Affymetrix microarrays to perform a technical validation of the RNA-Seq results. Statistically significant changes (p < 0.05) were detected in 186 transcripts, many of which are expressed at extremely low levels (5-10 copies/cell), which we confirmed through a separate spike-in control RNA-Seq experiment. Next, by fitting a linear model to exon-level RNA-Seq read counts, we detected signals of alternative splicing in 18 transcripts. Finally, we used the RNA-Seq data to identify differential expression (p < 0.0001) in eight previously unannotated regions that may represent novel transcripts. Overall, differentially expressed genes showed strong enrichment (p = 0.0002) for prior association with cardiovascular disease. At the network level, we found evidence for perturbation in pathways involving both cardiovascular system development and function as well as lipid metabolism.
Conclusions: We present a pilot study for transcriptome involvement in coronary artery calcification and demonstrate how RNA-Seq analyses using LCLs as a tissue surrogate may yield fruitful results in a clinical sequencing project. In addition to canonical gene expression, we present candidate variants from alternative splicing and novel transcript detection, which have been unexplored in the context of this disease.
Figures



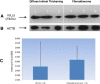

References
-
- Roberts R, McPherson R, Stewart AFR. In: Cardiovascular Genetics and Genomics. Roden D, editor. Oxford, UK: Wiley-Blackwell; 2009. Genetics of atherosclerosis; pp. 151–166.
-
- Bijnens AP, Lutgens E, Ayoubi T, Kuiper J, Horrevoets AJ, Daemen MJ. Genome-wide expression studies of atherosclerosis: critical issues in methodology, analysis, interpretation of transcriptomics data. Arterioscler Thromb Vasc Biol. 2006;15(6):1226–1235. doi: 10.1161/01.ATV.0000219289.06529.f1. - DOI - PubMed
-
- Skogsberg J, Lundstrom J, Kovacs A, Nilsson R, Noori P, Maleki S, Kohler M, Hamsten A, Tegner J, Bjorkegren J. Transcriptional profiling uncovers a network of cholesterol-responsive atherosclerosis target genes. PLoS Genet. 2008;15(3):e1000036. doi: 10.1371/journal.pgen.1000036. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

