Pooled analysis of the safety and tolerability of onabotulinumtoxinA in the treatment of chronic migraine
- PMID: 24628923
- PMCID: PMC4233954
- DOI: 10.1111/ene.12393
Pooled analysis of the safety and tolerability of onabotulinumtoxinA in the treatment of chronic migraine
Abstract
Background and purpose: OnabotulinumtoxinA was effective and well tolerated for prophylaxis of headache in patients with chronic migraine (CM) in two randomized, double-blind, placebo-controlled, phase 3 trials. To further assess the safety and tolerability of onabotulinumtoxinA in CM prophylaxis in adults, the pooled safety data from four double-blind, placebo-controlled trials were analyzed.
Methods: The pooled analysis included two phase 2 and two phase 3 double-blind, placebo-controlled trials. The safety population was 2436 patients, 1997 of whom received ≥1 dose of onabotulinumtoxinA. The studies shared similar dosing intervals (approximately 12 weeks) with doses between 75 and 260 U. Safety assessments included adverse events (AEs), physical examination and clinical laboratory tests.
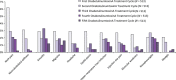
Results: OnabotulinumtoxinA was safe and well tolerated, with a low discontinuation rate (3.4%) due to AEs. The majority of patients in this pooled analysis received doses between 150 and 200 U, with an average of 163 U per treatment cycle. Of the 1997 patients who received any onabotulinumtoxinA injections, 1455 patients (72.9%) reported at least one AE. Neck pain (12.6%) was the most common onabotulinumtoxinA-associated AE, followed by muscle weakness (8.0%), musculoskeletal stiffness (6.1%) and eyelid ptosis (4.6%). Serious AEs were infrequent, occurring in 5.4% of patients who received any onabotulinumtoxinA treatment and 3.0% of patients receiving placebo. AEs were consistent with the known tolerability profile of onabotulinumtoxinA.
Conclusions: Multiple treatments with onabotulinumtoxinA at doses of 75-260 U administered every 12 weeks, and up to five treatment cycles, were well tolerated for the prophylaxis of headache in adults with CM.
Keywords: BOTOX; chronic migraine; onabotulinumtoxinA; safety; tolerability.
© The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of EAN.
Figures
Comment in
-
Can the immunological response to botulinum toxin trigger headaches?Neurotox Res. 2015 Jan;27(1):69-70. doi: 10.1007/s12640-014-9490-z. Epub 2014 Aug 14. Neurotox Res. 2015. PMID: 25119058
References
-
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. - PubMed
-
- Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71:559–566. - PubMed
-
- Buse DC, Manack A, Serrano D, Turkel C, Lipton RB. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry. 2010;81:428–432. - PubMed
-
- Dodick DW. Clinical practice. Chronic daily headache. N Engl J Med. 2006;354:158–165. - PubMed
-
- Natoli J, Manack A, Dean B, et al. Global prevalence of chronic migraine: a systematic review. Cephalalgia. 2010;30:599–609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical