Effects of varenicline, nicotine or placebo on depressive symptoms in postmenopausal smokers
- PMID: 24628943
- PMCID: PMC5068915
- DOI: 10.1111/j.1521-0391.2014.12130.x
Effects of varenicline, nicotine or placebo on depressive symptoms in postmenopausal smokers
Abstract
Background: Varenicline carries a black box warning for neuropsychiatric adverse events.
Objective: We examined varenicline use and past history of major depressive disorder (MDD) on depressive symptoms during smoking cessation.
Method: This is a secondary analysis of two smoking cessation studies in 152 postmenopausal women who received placebo or nicotine patch, or 78 women who received varenicline with relaxation. Lifetime history of MDD (LH-MDD) was assessed at baseline and women with current MDD were excluded. Center for Epidemiologic Study Depression scale (CESD) measured depressive symptoms at baseline, 6 and 12 weeks.
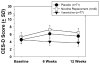
Results: Baseline CESD scores were 5.3 + 4.4. Those with a LH-MDD reported higher CESD scores (p > .001). Those taking varenicline reported lower scores over all time periods compared to nicotine or placebo (p < .01). The differences between varenicline and the other treatments remained when controlling for LH-MDD, indicating an independent effect. CESD scores were associated with concurrent smoking status (p < .001), and with withdrawal symptoms (p < .001).
Conclusion: CESD score were lower in those receiving varenicline, whether this is due to an anti-depressant effect, subject selection, use of relaxation or another cause is unknown. Varenicline does not increase depressive symptoms during smoking cessation in postmenopausal women without current MDD. Subjects with a LH-MDD are susceptible to developing depressive symptoms during smoking cessation, regardless of pharmacologic aid.
Scientific significance: Pharmacologic aids did not increase depression symptoms in this select population of postmenopausal women without current depression. Smoking cessation does increase depressive symptoms in those with LH-MDD, though the degree of increase was not clinically meaningful.
© American Academy of Addiction Psychiatry.
Conflict of interest statement
Declaration of Interest No competing financial interests exist.
Figures
References
-
- Hayatbakhsh MR, Najman JM, O’Callaghan MJ, et al. Association between smoking and respiratory function before and after menopause. Lung. 2011;189:65–71. - PubMed
-
- NIH State-of-the-Science Conference Statement on improving end-of-life care. NIH Consens State Sci Statements. 2004 Dec 6–8;21:1–26. - PubMed
-
- Copeland AL, Martin PD, Geiselman PJ, et al. Predictors of pretreatment attrition from smoking cessation among pre-and postmenopausal, weight-concerned women. Eat Behav. 2006;7:243–251. - PubMed
-
- Oncken C, Cooney J, Feinn R, et al. Transdermal nicotine for smoking cessation in postmenopausal women. Addict Behav. 2007;32:296–309. - PubMed
-
- Administration USFaD. Public Health Advisory: FDA Requires New Boxed Warnings for the Smoking Cessation Drugs Chantix and Zyban. Jul 01, 2009.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous