Host immune response to infection and cancer: unexpected commonalities
- PMID: 24629336
- PMCID: PMC3996827
- DOI: 10.1016/j.chom.2014.02.003
Host immune response to infection and cancer: unexpected commonalities
Abstract
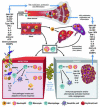
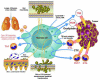
Both microbes and tumors activate innate resistance, tissue repair, and adaptive immunity. Unlike acute infection, tumor growth is initially unapparent; however, inflammation and immunity affect all phases of tumor growth from initiation to progression and dissemination. Here, we discuss the shared features involved in the immune response to infection and cancer including modulation by commensal microbiota, reactive hematopoiesis, chronic immune responses and regulatory mechanisms to prevent collateral tissue damage. This comparative analysis of immunity to infection and cancer furthers our understanding of the basic mechanisms underlying innate resistance and adaptive immunity and their translational application to the design of new therapeutic approaches.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Anderson AC. Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol. 2012;24:213–216. - PubMed
-
- Ascensao JL, Oken MM, Ewing SL, Goldberg RJ, Kaplan ME. Leukocytosis and large cell lung cancer. A frequent association. Cancer. 1987;60:903–905. - PubMed
-
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

