Behavioral cessation treatment of waterpipe smoking: The first pilot randomized controlled trial
- PMID: 24629480
- PMCID: PMC4141480
- DOI: 10.1016/j.addbeh.2014.02.012
Behavioral cessation treatment of waterpipe smoking: The first pilot randomized controlled trial
Abstract
Background: Waterpipe use has increased dramatically in the Middle East and other parts of the world. Many users exhibit signs of dependence, including withdrawal and difficulty quitting, but there is no evidence base to guide cessation efforts.
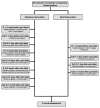
Methods: We developed a behavioral cessation program for willing-to-quit waterpipe users, and evaluated its feasibility and efficacy in a pilot, two arm, parallel group, randomized, open label trial in Aleppo, Syria. Fifty adults who smoked waterpipe ≥3 times per week in the last year, did not smoke cigarettes, and were interested in quitting were randomized to receive either brief (1 in-person session and 3 phone calls) or intensive (3 in-person sessions and 5 phone calls) behavioral cessation treatment delivered by a trained physician in a clinical setting. The primary efficacy end point of the developed interventions was prolonged abstinence at three months post-quit day, assessed by self-report and exhaled carbon monoxide levels of <10 ppm. Secondary end points were 7 day point-prevalent abstinence and adherence to treatment.
Results: Thirty percent of participants were fully adherent to treatment, which did not vary by treatment group. The proportions of participants in the brief and intensive interventions with prolonged abstinence at the 3-month assessment were 30.4% and 44.4%, respectively. Previous success in quitting (OR=3.57; 95% CI=1.03-12.43) predicted cessation. Higher baseline readiness to quit, more confidence in quitting, and being unemployed predicted a better adherence to treatment (all p-values <0.05).
Conclusions: Brief behavioral cessation treatment for waterpipe users appears to be feasible and effective.
Keywords: Behavioral treatment; Cessation; Randomized controlled trial; Waterpipe.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
References
-
- Asfar T, Weg MV, Maziak W, Hammal F, Eissenberg T, Ward KD. Outcomes and adherence in Syria’s first smoking cessation trial. American Journal of Health Behavior. 2008;32:146–156. - PubMed
-
- Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Revealing the multidimensional framework of the Minnesota nicotine withdrawal scale. Current Medical Research and Opinion. 2005;21:749–760. - PubMed
-
- DiClemente CC, Prochaska JO, Fairhurst SK, Velicer WF, Velasquez MM, Rossi JS. The process of smoking cessation: An analysis of precontemplation, contemplation, and preparation stages of change. Journal of Consulting and Clinical Psychology. 1991;59:295–304. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical